
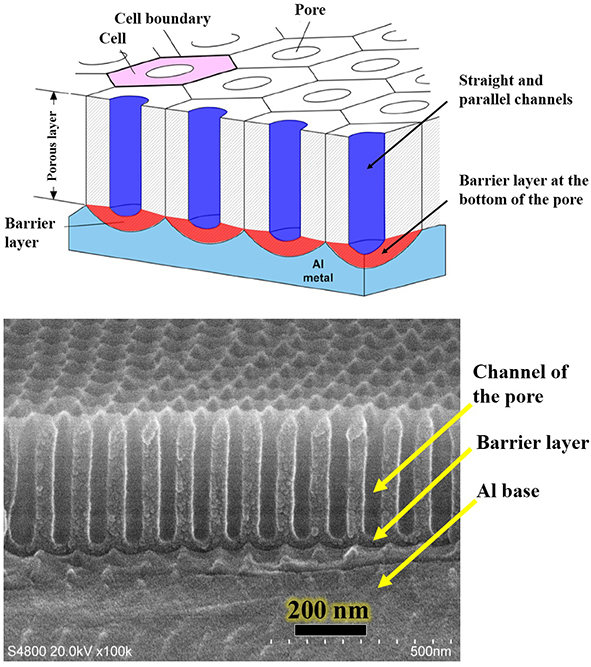
图1. 铝基AAO模板(多孔纳米模板)结构示意图及SEM图
(注:为了简化模型,图1上部示意图中,AAO的正面画成了绝对平整,实际上在每个孔四周都有凸起,在微观上表面是不平整的)
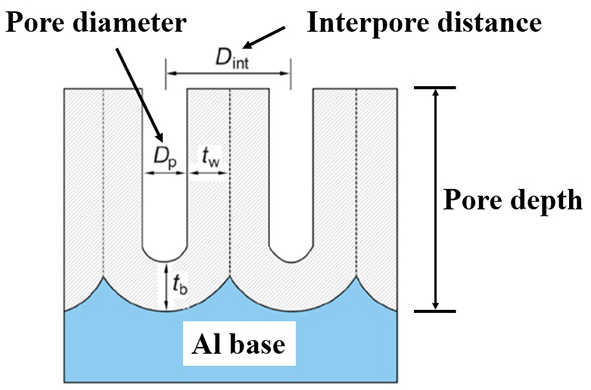
图2. 铝基AAO模板或多孔纳米模板的截面示意图
铝基AAO(Anodic Aluminum Oxide)模板,又叫单通AAO模板,它具有蜂窝状结构(如图1所示),由许多六角形柱体氧化铝(Al2O3)原胞组成,每个原胞中间有个圆形的小孔,在孔的下端有个半球形的阻挡层,阻挡层下方为铝基,也可以称之为单通AAO或单通AAO模板。铝基AAO是多孔纳米模板系列最为简单的一个,制备容易,成品率高,可实现几百平方厘米以上的大面积均匀制备。图2为铝基AAO模板或多孔纳米模板的截面示意图。根据制备条件的不同,单通AAO的孔径可以实现孔径几十纳米到几百纳米,孔深(即AAO模板上面AAO的厚度)连续可控,浅则几十纳米,深可以达到几十微米。铝基AAO表面纳米级孔洞排列短程有序,有序范围为微米级,排列方式为六角密排结构。其孔径大面积范围内均匀可控,可作为母版将多孔结构转移到其它材料之上。高密度的孔分布以及细长的孔道,提供了一种比表面极高的三维纳米结构,可以实现有机分子的大量吸附,在比如光学检测等方面有很好的应用前景。

图3. 铝基AAO(多孔纳米模板)的SEM图
图3左侧为几种典型的铝基AAO的SEM图的俯视图,右侧为其截面视角SEM图,可见AAO模板在小范围内孔呈六角密排,孔径比较均匀,孔底部阻挡层清晰可见。

图4.铝基AAO模板(多孔纳米模板)产品实物图
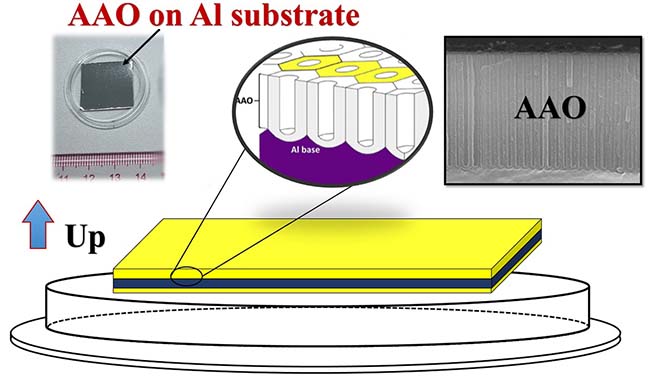
图5. 铝基AAO产品放置方向示意图
铝基AAO模板一面为高度有序多孔结构。由于有铝基底的支撑,铝基AAO强度较高,可以用镊子夹取和略微折弯,可以进行短时间的超声清洗。注意切勿用手指直接接触膜表面,否则手上的油脂或其它异物容易沾到膜表面,而且非常难以将其清洗干净。
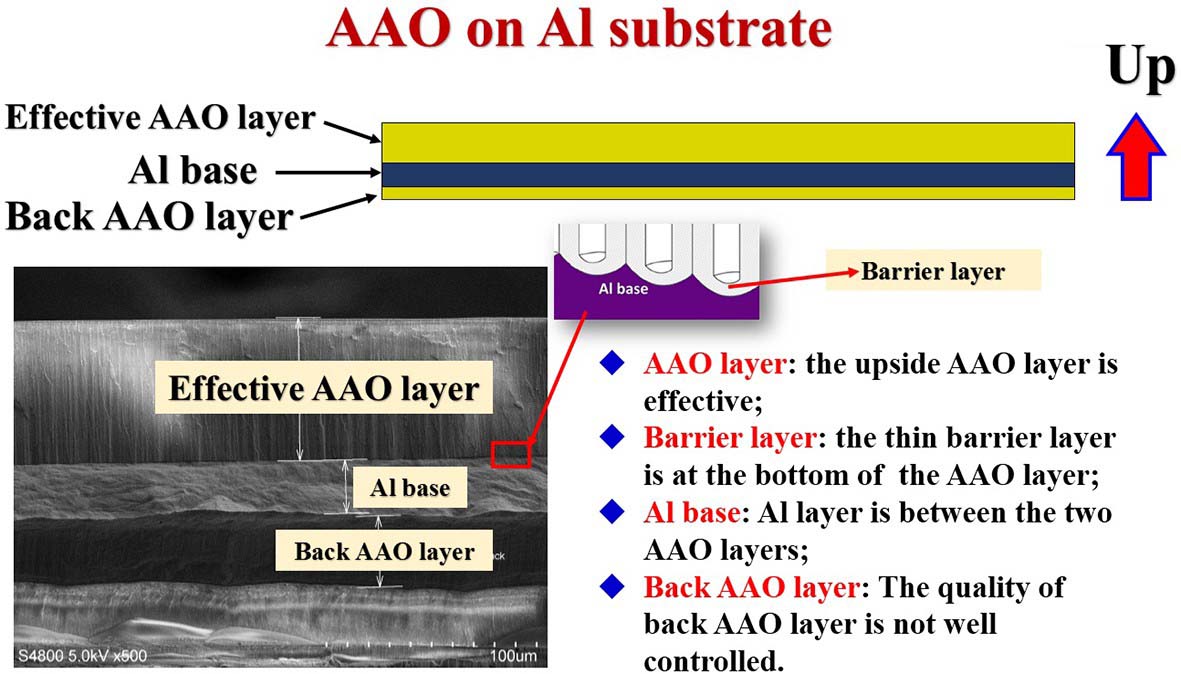
图6. 铝基AAO模板结构示意图
铝基AAO模板产品包装盒内正面朝上放置,上表面为有效AAO层,产品背面也有一层多孔氧化铝,只不过这层氧化铝较有效AAO层薄一些,质量差一些,请不要使用这层氧化层作为多孔模板。铝基单通AAO模板放置方式是正面向上朝着盒盖,如果两面外观有明显不同的话,正面显得光亮均匀一些,反面粗糙不均匀一些,如果两面外观上一模一样,则无法通过肉眼区分,所以为了防止混淆正反面,建议在取出单通AAO的时候就用记号笔在其背面做好标记。AAO膜可以采用5wt%的磷酸进行慢速去除,或采用5~10wt.%的NaOH进行快速去除(在确保AAO部分与磷酸或NaOH容易能够直接接触的前提下)。
关于如何除去铝基:如果使用铝基AAO作为模板制备高分子纳米线或纳米棒,可以将高分子的溶液滴在AAO表面,或者将高分子薄膜铺在AAO表面然后加热。制备结束后,由于AAO的正面被高分子层保护,所以不可以将整个样品简单放在NaOH中来去除AAO。此时可以用NaOH去除背面的氧化层,然后用氯化铜除去铝基,最后用NaOH快速去除AAO模板层。
除铝基详细操作指导:我们的单通AAO和V形AAO产品表面是没有任何高分子材料的。这里所说的是,如果您使用单通或V型AAO的过程中,如果在其表面涂覆了一层其它材料,比如高分子材料,由于这一层材料的覆盖,如果将单通AAO直接放在NaOH溶液中,这一层材料将会把AAO与NaOH溶液隔离,从而不能达到溶解AAO的目的。由于单通AAO正面涂覆了高分子材料,因此不能直接用NaOH溶液来去除AAO模板,因为此时AAO与NaOH被高分子层隔离了。所以必须从背面去除AAO。
(1) 去除背面氧化层:将样品正面朝上,轻轻放置在质量分数为5%的NaOH溶液的表面(常温下),由于高分子层一般都是疏水的,所以样品会漂浮在溶液表面(当然,沉下去也没事,不影响),NaOH会很快(几分钟到十几分钟)将背面的氧化铝层溶解,从而铝基露出来,露出的铝基会与NaOH直接接触,生产氢气,可以看到很多气泡生产,说明背面氧化铝除完了。由于反应不是太剧烈,样品一般不会被冲起来。将样品取出后用水冲洗。
(2) 去除背面铝基:由于NaOH跟铝反应非常非常慢,所以不用NaOH除铝。配制氯化铜(CuCl2)盐酸溶液,如果没有氯化铜的话可以用硫酸铜,溶液浓度可以随意,原理是氯化铜和盐酸浓度越高,反应越快,越剧烈,时间越短,比如,可以用质量分数20%的氯化铜,盐酸体积浓度可以为10%。将样品置于溶液表面,此时,铜离子、氢离子、铝会发生反应,生产氢气、铜单质、铝离子,当氯化铜浓度高时,反应非常剧烈,产生大量的热,反应很剧烈时,气泡会把样品顶起来剧烈抖动。如果感觉反应太剧烈了,可以立刻往溶液里倒去离子水稀释溶液,反应速度马上就会下降。如果感觉反应太慢了,可以往溶液里加一些氯化铜和盐酸。等气泡消失的时候,铝也反应完了,样品变得透明(假如高分子层是无色透明的)。此时可以将样品取出再放在一杯新的氯化铜盐酸溶液表面十分钟,让残余的肉眼看不见的铝彻底去除干净。将样品取出用水轻轻清洗。
(3) 将样品正面朝上漂浮在质量分子5%的NaOH溶液表面(常温下),一般情况下,厚度5微米以下的单通AAO,十几二十分钟就可以完全消失,如果厚度几十微米,可以适当延长时间到三十分钟甚至1小时,由于NaOH跟AAO反应无气泡生产,而且颜无变化,所以肉眼观察起来不能判断AAO是否完全去除干净,最终还要以SEM检测为准。
提示:当正面AAO裸露时,去除背面AAO的方法:将AAO漂浮于NaOH溶液表面,但是需要首先将样品正的四周涂覆少量的疏水材料,比如采用指甲油,或者直接用高温胶带粘住正面四周边缘,涂覆或粘贴宽度1~3mm即可,目的是在漂浮的时候防止NaOH溶液蔓延到正面的AAO上。当AAO膜很薄(比如如小于2微米),而且需要去除铝基的时候,不建议AAO表面裸露,因为在去除铝基的时候产生的气泡很可能冲破AAO薄膜,所以正面最好是涂覆十几到几十微米的高分子保护膜。我们不建议您对表面未涂保护膜层的单通AAO进行去铝基操作。
铝基AAO模板可以采用SEM或AFM对膜进行表征。由于AAO本身不导电,所以当AAO的厚度超过几微米时,在SEM测试时请进行喷金或喷碳处理。当AAO厚度小于1微米时,可以直接观察,无需喷金或喷碳。截面观察时,可以将膜轻轻折弯成90°,弯折处形成新鲜断面可供观测。当单通AAO模板的孔径与孔间距差别很小的时候,弯折处很难形成可供观测的截面,弯折法不适用。当AAO的厚度达到几十微米时,弯折法同样有局限,因为弯折后形成的裂口比较小,观测时AAO断面底部可能会被挡住一部分,此时应该把弯折角度变大,或者将其折断来观察断面。单通AAO厚度在测量的时候存在一定的误差,由于测试角度等原因,而且膜本身也有一定的厚度波动,测试有时误差可达10%,因此膜厚的非常精确的值需要您自行检测。
温馨提示:AAO模板为自下而上的方法制备,属于自组织结构,因此它的孔径都有一定的分布范围,而不是单一值,特别是孔间距450nm的模板不均匀性大一些。孔的排列为短程有序(微米级),每个有序区域可称为一个“筹”,在筹边界处孔的形状可能大都不是正圆形。超薄膜的孔径分布比双通厚膜以及单通膜宽一些。如果您对多孔膜的孔径要求均匀程度非常高,对孔的圆形程度要求非常高,那么AAO并不是好的选择。
对于某一个孔中心间距的AAO(比如孔间距450nm)来说,孔越大,孔的形状约接近于圆形,孔越小,孔的形状越偏离圆形;规格列表中孔深其实就是有效AAO的厚度,不包括背面氧化层以及中间铝层的厚度。总的厚度约为0.15~0.2mm。
关于切割:单通AAO可以切成更小的片使用,一般不用剪刀来剪,剪刀容易造成样品弯曲和膜的开裂。对于膜厚不大于5微米的单通AAO,可以找一个平的桌面或一块玻璃,铺上一块无尘布,然后将单通AAO反扣在无尘布上,用直尺和美工刀在背面划切即可,不需要太用力。可以多划几刀切掉,也可以在快切掉的时候停止,然后来回掰几次就会断开。
注意:当单通AAO膜厚为40~60微米时,切割时切割线两边会出现很多裂纹,而且裂纹会扩展。所以在切割的时候不能在背面切割,而要从正面切割,即AAO正面向上用无尘布垫在AAO表面,尺子压在无尘布上,沿着直尺,用新鲜的美工刀刀尖来回划刻,一定不要用力压,而是轻轻地来回划刻,就像锯木头一样,划几十次之后表层的AAO就断了,然后再接着划几刀,之后再来回掰几次就可以掰断了。即使是这样操作,断口两侧仍然会有很少了的裂纹,请知悉。
单通AAO可以放心超声清洗,可以使 用丙酮、乙醇、异丙醇、去离子水清洗。
注:测单通AAO的截面的SEM之前,建议喷金或喷碳以增加导电性,有利于截面的清晰观察。
1. 手机淘宝(官方淘宝店铺:科学材料站)
2. 点击进入淘宝网页链接
BSP 单通AAO模板 (膜厚: 500nm) | |||
产品代码 | 产品描述 (孔径/孔中心间距) | 产品价格及规格 | 库存状态 |
| 31101045 | 70nm/100nm | ¥170 (20*20mm) | 请咨询 |
| 31101061 | 50nm/125nm | ¥170 (20*20mm) | 请咨询 |
| 31101062 | 70nm/125nm | ¥170 (20*20mm) | 请咨询 |
| 31101063 | 100nm/125nm | ¥170 (20*20mm) | 请咨询 |
| SCI Materials Hub is Committed to Offering The Best Price & Customer Services! | |||
*大量需求请联系我们咨询更多折扣
**可提供13%增值税专用发票
3. 其它联系方式
电话:+86 130-0303-8751/+86 156-0553-2352
微信:SCI-Materials-Hub
如需报价单请联系:Email: contact@scimaterials.cn
部分使用和引用我们材料的参考文献 (摘自于Google Scholar)
二氧化碳还原
1. ACS Nano Strain Relaxation in Metal Alloy Catalysts Steers the Product Selectivity of Electrocatalytic CO2 Reduction
The bipolar membrane (Fumasep FBM) in this paper was purchased from SCI Materials Hub, which was used in rechargeable Zn-CO2 battery tests. The authors reported a strain relaxation strategy to determine lattice strains in bimetal MNi alloys (M = Pd, Ag, and Au) and realized an outstanding CO2-to-CO Faradaic efficiency of 96.6% with outstanding activity and durability toward a Zn-CO2 battery.
2. Front. Chem. Boosting Electrochemical Carbon Dioxide Reduction on Atomically Dispersed Nickel Catalyst
In this paper, Vulcan XC-72R was purchased from SCI Materials Hub. Vulcan XC 72R carbon is the most common catalyst support used in the anode and cathode electrodes of Polymer Electrolyte Membrane Fuel Cells (PEMFC), Direct Methanol Fuel Cells (DMFC), Alkaline Fuel Cells (AFC), Microbial Fuel Cells (MFC), Phosphoric Acid Fuel Cells (PAFC), and many more!
3. Adv. Mater. Partially Nitrided Ni Nanoclusters Achieve Energy-Efficient Electrocatalytic CO2 Reduction to CO at Ultralow Overpotential
An AEM membrane (Sustainion X37-50 Grade RT), purchased from SCI Materials Hub) was activated in 1 M KOH for 24 h, washed with ultra-purity water prior to use.
4. Adv. Funct. Mater. Nanoconfined Molecular Catalysts in Integrated Gas Diffusion Electrodes for High-Current-Density CO2 Electroreduction
In this paper (Supporting Information), an anion exchanged membrane (Fumasep FAB-PK-130 obtained from SCI Materials Hub (www.scimaterials.cn)) was used to separate the catholyte and anolyte chambers.
SCI Materials Hub: we also recommend our Fumasep FAB-PK-75 for the use in a flow cell.
5. Appl. Catal. B Efficient utilization of nickel single atoms for CO2 electroreduction by constructing 3D interconnected nitrogen-doped carbon tube network
In this paper, the Nafion 117 membrane was obtained from SCI Materials Hub.
In this paper, Proton exchange membrane (Nafion 117), Nafion D520, and Toray 060 carbon paper were purchased from SCI Materials Hub.
7. National Science Review Confinement of ionomer for electrocatalytic CO2 reduction reaction via efficient mass transfer pathways
An anion exchange membrane (PiperION-A15-HCO3) was obtained from SCI Materials Hub.
8. Catalysis Communications Facilitating CO2 electroreduction to C2H4 through facile regulating {100} & {111} grain boundary of Cu2O
Carbon paper (TGPH060), membrane solution (Nafion D520), and ionic membrane (Nafion N117) were obtained from Wuhu Eryi Material Technology Co., Ltd.
Note: Wuhu Eryi Material Technology Co. is a company hold by SCI Materials Hub.
9. Advanced Energy Materials Interatomic Electronegativity Offset Dictates Selectivity When Catalyzing the CO2 Reduction Reaction
The bipolar membrane (Fumasep FBM), carbon paper (SIGRACET 29BC, Freudenberg paper H23C2), ion exchange membrane (Nafion N117), and anion exchange membrane (Fumasep, FAA-3-PK-130) were all obtained from SCI Materials Hub.
10. Separation and Purification Technology *CO spillover induced by bimetallic xZnO@yCuO active centers for enhancing C–C coupling over electrochemical CO2 reduction
5 % Nafion solution was obtained through SCI Materials Hub.
11. National Science Review Confinement of ionomer for electrocatalytic CO2 reduction reaction via efficient mass transfer pathways
In this paper, PiperION-A5-HCO3 anion exchange resin, Fumion FAA anion exchange resin, PiperION-A15-HCO3 and FAA-3-50 were purchased from SCI Materials Hub.
12. Vacuum Controllable dual Cu–Cu2O sites derived from CuxAl-LDH for CO2 electroreduction to hydrocarbons
Nafion and carbon paper (TGPH060) were supplied through SCI Materials Hub.
13. Chemical Engineering Journal Coupling electrocatalytic CO2 reduction with glucose oxidation for concurrent production of formate with high efficiency
An AEM membrane (PiperION, purchased from SCI Materials Hub) was activated in 1 M KOH for 24 h, washed with ultra-purity water prior to use.
In this paper, Sustainion X37-50 Grade RT membrane and the MEA electrolyzer (CRRMEA1a, Figure S34) with 1cm2 active area were obtained from SCI Materials Hub.
微信公众号中文报道:Chem:基于热力学驱动的混合策略形成Cu0/Cu+/Cu0界面用于中性条件CO2电还原C2+
15. Surfaces and Interfaces Modulating surface microenvironment based on Ag-adorned CuO flower-liked nanospheres for strengthening C-C coupling during CO2RR
5 wt.% of Nafion solution, and N115 proton exchange membrane were procured with the help of SCI Materials Hub
16. ACS Appl. Energy Mater. Nanoporous Bismuth Induced by Surfactant-Modified Dealloying for Efficient Electrocatalytic Reduction of CO2 to Formic Acid
The anion exchange membrane (AEM, PiperION A20) and cation exchange membrane (CEM, Nafion 117) were obtained from SCI Materials Hub.
17. Adv. Energy Mater. Tailoring Microenvironments and In Situ Transformations of Cu Catalysts for Selective and Stable Electrosynthesis of Multicarbon Products
For GDE-based CO₂ electrolysis, the MEA reactor (CRRMEA5a, Sci-Materials Hub) consists of a titanium anode plate and a cathode plate with flow fields, along with insulating gaskets, integrated into a compression cell. The geometric area of each flow field is 5 cm² An anion exchange membrane (PiperION, A40-HCO3, Versogen) was used to separate the anode and the cathode.
18. Journal of Environmental Chemical Engineering Evaluation of electromethanogenesis in a microbial electrolysis cell using nylon cloth as a separator: reactor performance and metagenomic analysis
A commercial Nafion PEM (SCI Materials Hub) was used as the control to compare the electromethanogenesis performance.
CRRMEA1a 1cm2 MEA electrolyzer (Figure 4d) was obtained from SCI Materials Hub.
微信公众号中文报道:安徽师范大学最新Angew!全pH范围内铋基催化剂用于安培级电流密度电催化CO2还原
20. Separation and Purification Technology Coupling regulation of boron doping and morphology in nano-floral CuO using one pot method for electrocatalytic CO2 reduction
Carbon paper (TGPH060), Dupont Nafion solution (D520), and proton exchange membrane (N117) were acquired by SCI Materials Hub.
21. Chemical Engineering Journal Manipulating dual effects of morphology and oxygen vacancies through the incorporation of CuO onto CeO2 nanospheres for electrochemical CO2 reduction
Carbon paper (TGPH060), Dupont Nafion solution (D520), and proton exchange membrane (N117) were acquired by SCI Materials Station Hub (SCI Materials Hub, the same author as Ref. 20).
22. Advanced Materials Universal Formation of Single Atoms from Molten Salt for Facilitating Selective CO2 Reduction
The Nafion D520 dispersion and gas diffusion electrode (GDE, Sigracet, 39BB) were obtained from SCI Materials Hub (www.scimaterials.cn).
23. Science Bulletin Compressive strain in Cu catalysts: Enhancing generation of C2+ products in electrochemical CO2 reduction
CRRMEA1a 1cm2 MEA electrolyzer (Figure 3a) was obtained from SCI Materials Hub.
微信公众号中文报道:Science Bulletin:优化水覆盖度促进安培级电流密度下CO2还原C2+
Carbon paper (SGL CARBON, SGL36BB, purchased from SCI Materials Hub)
25. Chemical Engineering Journal In-situ reconstruction of active bismuth for enhanced CO2 electroreduction to formate
Anion-exchange membrane (Fumasep FAA-3–50) was purchased from SCI Materials Hub.
26. Nature Communications Bromide-mediated membraneless electrosynthesis of ethylene carbonate from CO2 and ethylene
Nafion D520 polymer binder solution (5 wt%) was purchased from SCI Materials Hub.
27. Ionics Sulfur-modified promoting the electrochemical CO2 reduction into formate performance of BiOI
Carbon black (Vulcan XC- 72, Cabot), teflon-treated carbon paper (TGP-H- 060, Toray), and proton exchange membrane (Nafion- 117) were purchased from SCI Materials Hub.
28. Nature Communications Continuous decoupled redox electrochemical CO2 capture
The anion exchange membrane (FAA-3-PK-75) with a thickness of 75 ± 5 μm was purchased from SCI Materials Hub.
29. Journal of Energy Chemistry Construction of efficient electrodes for CO2RR through microenvironment regulation of hydrophobic ionomer
An anion exchange membrane (PiperION-A15-HCO3, SCI Materials Hub) was used to separate the anode and cathode chambers.
30. Journal of Energy Chemistry The insights into ionomer-catalyst interactions enabling high-efficiency CO2 electroreduction in pure water
Methylimidazolium ionomer powder (Sustainion XA-9, dispersed in ethanol at 5 wt%), and piperidinium ionomer solution (PiperION A5, dispersed in ethanol at 5 wt%) were purchased from SCI Materials Hub (
31. ChemElectroChem Scaling CO Electrolyzers for Carbon-Neutral Chemical Synthesis
Specifically, the PTFE-CB ink was prepared by mixing 0.1 g of CB (Vulcan XC-72R, SCI Materials Hub) with PTFE (25 % of the CB mass) in 50 mL of methanol. After ultrasonication for 2 hours, the ink was air-sprayed onto the gas diffusion layer (GDL) (Sigracet 28 BC, SCI Materials Hub) at 60 °C. The anion exchange membrane (AEM) was Fumasep FAA-3-50 (SCI Materials Hub)
32. American Institute of Chemical Engineers Porous carbon-supported Ag catalysts enablehigh-current-density CO2 electroreductionto CO with enhanced mass transport
Carbon paper (SGL Sigracet 39 BB SGL Carbon), anion exchange membrane (Sustainion X37-50 Grade T, Dioxide Materials), Ni foam was obtained from SCI Materials Hub.
33. Applied Catalysis B: Environment and Energy Microenvironmental hydrophobicity-regulated evolution of reaction sites for boosting electrochemical CO2 reduction
A gas diffusion layer AvCarb GDS2240 was obtained from SCI MATERIALS HUB.
34. Applied Catalysis B: Environment and Energy Boosting seawater desalination driven by electroreduction of CO2 to formate on bimetallic Sn/Cu polyphthalocyanine frameworks
Gas diffusion layers (GDLs) consisting of Sigracet 36BB substrates (SCI Materials Hub) were utilized.
35. Catalysis Today Efficient and cost-effective electrocatalysts for coproduction of formate through electrocatalytic oxidation of PET-derived ethylene glycol coupled with CO2 reduction
Cation exchange membrane ((Nafion 117, DuPont) was purchased from SCI Materials Hub, China.
电池
1. J. Mater. Chem. A Blocking polysulfides with a Janus Fe3C/N-CNF@RGO electrode via physiochemical confinement and catalytic conversion for high-performance lithium–sulfur batteries
Graphene oxide (GO) in this paper was obtained from SCI Materials Hub. The authors introduced a Janus Fe3C/N-CNF@RGO electrode consisting of 1D Fe3C decorated N-doped carbon nanofibers (Fe3C/N-CNFs) side and 2D reduced graphene oxide (RGO) side as the free-standing carrier of Li2S6 catholyte to improve the overall electrochemical performance of Li-S batteries.
This paper used more than 10 kinds of materials from SCI Materials Hub and the authors gave detailed properity comparsion.
The commercial IEMs of Fumasep FAB-PK-130 and Nafion N117 were obtained from SCI Materials Hub.
Gas diffusion layers of GDL340 (CeTech) and SGL39BC (Sigracet) and Nafion dispersion (Nafion D520) were obtained from SCI Materials Hub.
Zn foil (100 mm thickness) and Zn powder were obtained from the SCI Materials Hub.
Commercial 20% Pt/C, 40% Pt/C and IrO2 catalysts were also obtained from SCI Materials Hub.
3. Journal of Energy Chemistry Vanadium oxide nanospheres encapsulated in N-doped carbon nanofibers with morphology and defect dual-engineering toward advanced aqueous zinc-ion batteries
In this paper, carbon cloth (W0S1011) was obtained from SCI Materials Hub. The flexible carbon cloth matrix guaranteed the stabilization of the electrode and improved the conductivity of the cathode.
4. Energy Storage Materials Defect-abundant commercializable 3D carbon papers for fabricating composite Li anode with high loading and long life
The 3D carbon paper (TGPH060 raw paper) were purchased from SCI Materials Hub.
5. Nanomaterials A Stable Rechargeable Aqueous Zn–Air Battery Enabled by Heterogeneous MoS2 Cathode Catalysts
Nafion D520 (5 wt%), and carbon paper (GDL340) were received from SCI-Materials-Hub.
Carbon cloth (W0S1011) and other electrochemical consumables required for air cathode were provided by SCI Materials Hub.
The Zn sheet (99.99%) was purchased from SCI Materials Hub.
8. Nature Communications Atomic-scale regulation of anionic and cationic migration in alkali metal batteries
The lithium metal disk (purity: 99.9%, diameter: 16 mm, thickness: 0.6 mm) was obtained from SCI Materials Hub.
9. Chemical Engineering Journal Zinc-based energy storage with functionalized carbon nanotube/polyaniline nanocomposite cathodes
CNTs were purchased from SCI Materials Hub.
10. ACS Nano Interfacial Chemistry Modulation via Amphoteric Glycine for a Highly Reversible Zinc Anode
Zn foil (>99.99%, 100 μm) was purchased from SCI Materials Hub.
11. ACS Nano High-Energy and Long-Lived Zn–MnO2 Battery Enabled by a Hydrophobic-Ion-Conducting Membrane
Zn foil (99.9%), carbon paper, and carbon felt were obtained from SCI Materials Hub.
12. Nature Communications Unravelling rechargeable zinc-copper batteries by a chloride shuttle in a biphasic electrolyte
Carbon cloth (CeTech W0S1011), PP membrane (Celgard 2300), Glass fiber (Whatman GF/A), anion exchange membrane (Fumasep FAB-PK-130), and cation exchange membrane (Nafion N-117) were purchased from sci materials hub.
13. PROCEEDINGS OF SPIE A dendrite-free and corrosion-suppressive metallic Zn anode regulated by the hybrid aqueous/organic electrolyte
Zn foil (99.9%, 100 μm thickness) was obtained from the SCI Materials Hub.
14. Journal of Alloys and Compounds Cr-induced enhancement of structural stability in δ-MnO2 for aqueous Zn-ion batteries
The Zn sheet (99.99%) and Whatman GF/D paper were available for purchase on on the SCI Materials Hub.
Carbon coating aluminum foils with a thickness of 16 µm were acquired from SCI Materials Hub.
16. Journal of Industrial and Engineering Chemistry Investigation into electrochemical catalytic properties and electronic structure of Mn doped SrCoO3 perovskite catalysts
KB-EC600JD superconducting carbon black was obtained from SCI Materials Hub.
17. Journal of Alloys and Compounds Inhibiting polysulfide shuttle and enhancing polysulfide redox: Conductive 2D metal-organic framework coated separators for lithium-sulfur batteries
Ketjen black was obtained from SCI Materials Hub
18. Chemical Engineering Journal Regulating N-doped biochar with Fe-Mo heterojunctions as cathode in long-life zinc-air battery
Conductive carbon black (Vulcan XC-72R) was purchased from SCI Materials Hub (Wuhu, Anhui).
19. Nature Portfolio Fast-kinetics and high-compatibility aqueous cadmium-metal battery for next-generation energy storage infrastructures (Under review.)
Cd foil (99.9%), Zn foil (99.9%) and polytetrafluoroethylene (PTFE) aqueous dispersion solution were obtained from the supplier of SCI Materials Hub.
All cells were assembled using two-electrode Swagelok-type configurations, supported by SCI Materials Hub.
Celgard 2400 separator (polypropylene (PP), 25 μm) was sourced from SCI Materials Hub.
21. ACS Applied Energy Materials 3D Graphene Nanoflake/Vertically Aligned Carbon Nanotube/CoAl Layered Double Oxide Composites for High-Performance Lithium-Ion Batteries
Carbon-coated aluminum foil was procured from SCI Materials Hub.
22. Chemistry A European Jouranl Regulating the Interfacial Charge Density by Constructing a Novel Zn Anode-Electrolyte Interface for Highly Reversible Zn Anode
Ketjenblack (KB) was purchased from SCI Materials Hub
23. Advanced Functional Materials A Biphasic Membraneless Zinc-Iodine Battery with High Volumetric Capacity
Graphite felt (GF, G650A) was purchased from SCI Materials Hub.
24. Science Advances Alcohol molecule coupling: A universal approach to modulating amorphousness in vanadium-based cathodes for high-rate and durable aqueous zinc-ion batteries
Fueiceel® BM03 Battery test hardware in Figure 6F was obtained from SCI Materials Hub. The Fueiceel® BM03 scale-up battery powering an LED panel.
25. Nature Communications Cooperative Jahn-Teller effect and engineered long-range strain in manganese oxide/graphene superlattice for aqueous zinc-ion batteries
The Zn||MnO2 coin cells were assembled by Zn foil (thickness of 0.1 mm, 99.99%, SCI Materials Hub) counter electrodes
26. Adv. Funct. Mater. Construction of Ionic Conductive Electrode/ Electrolyte Interphases via Li+ Coordination Regulator for 4.7 V Li/ LiNi0.9Co0.05Mn0.05O2 Batteries
The CR2032 coin cells were assembled with a Celgard 2400 separator (SCI Materials Hub.) in a glove box.
27. Journal of Energy Storage Bacterial cellulose mixed with glass fiber as a separator to achieve long cycle life of aqueous zinc-ion batteries
304 stainless steel foil (99.99 % purity, purchased from SCI Materials Hub)
28. Nature Communications Dual-structure-breaking electrolyte enables practical cadmium-metal battery
Two-electrode (Swagelok2E002) and three-electrode (Swagelok3E2e) Swagelok-type configurations were supported by SCI Materials Hub (Supplementary Figs. 102, 103)
The stacked configuration (BM03) for the scaled-up cell was supported by SCI Materials Hub.
Cd foil (99.9%), Zn foil (99.9%), and polytetrafluoroethylene (PTFE) aqueous dispersion solution (D-210C, solid content: 60 w%, size: ~0.25 μm size) were obtained from the supplier of SCI Materials Hub.
29. Nature Nanotechnology A nanoengineered lithium-hosting carbon/zinc oxide composite electrode material for efficient non-aqueous lithium metal batteries
The operando battery pressure sensing equipment (BS01) was purchased from SCI Materials Hub (www.scimaterials.cn).
Nafion 115 membrane (N115) was purchased from SCI Materials Hub.
电解水
1. International Journal of Hydrogen Energy Gold as an efficient hydrogen isotope separation catalyst in proton exchange membrane water electrolysis
The cathodic catalysts of Pt/C (20 wt%, 2–3 nm) and Au/C (20 wt%, 4–5 nm) were purchased from SCI Materials Hub.
2. Small Science Silver Compositing Boosts Water Electrolysis Activity and Durability of RuO2 in a Proton-Exchange-Membrane Water Electrolyzer
Two fiber felts (0.35 mm thickness, SCI Materials Hub) were used as the porous transport layers at both the cathode and the anode.
3. Advanced Functional Materials Hierarchical Crystalline/Amorphous Heterostructure MoNi/NiMoOx for Electrochemical Hydrogen Evolution with Industry-Level Activity and Stability
Anion-exchange membrane (FAA-3-PK-130) was obtained from SCI Materials Hub.
4. Chemical Engineering Journal Electronic configuration of single ruthenium atom immobilized in urchin-like tungsten trioxide towards hydrazine oxidation-assisted hydrogen evolution under wide pH media
The non-reinforced anion exchange membrane (AEM) of the coupled system was obtained from SCI Materials Hub (Fumasep FAA-3-50).
5. Cell Reports Physical Science Non-layered dysprosium oxysulfide as an electron-withdrawing chainmail for promoting electrocatalytic oxygen evolution
Nickel foam (NF) was offered by SCI Materials Hub (Wuhu, China), and was ultrasonicated in HCl solution, ethanol, and acetone in proper order before being used in electrochemical measurements.
6. Materials Today Catalysis Valence engineering via double exchange interaction in spinel oxides for enhanced oxygen evolution catalysis
Commercial Cu foam was purchased from SCI Materials Hub.
7. Advanced Functional Materials Elucidating the Critical Role of Ruthenium Single Atom Sites in Water Dissociation and Dehydrogenation Behaviors for Robust Hydrazine Oxidation-Boosted Alkaline Hydrogen Evolution
The nonreinforced anion exchange membrane (AEM) of the HzOR-assisted OWS system was purchased from SCI Materials Hub (Fumasep FAA-3-50).
8. ACS Omega Boosting Hydrogen Evolution through the Interface Effects of Amorphous NiMoO4–MoO2 and Crystalline Cu
Pt/C (20 wt %) was purchased from SCI Materials Hub.
9. Journal of Colloid and Interface Science The Dual Active Sites Reconstruction on Gelatin In-Situ Derived 3d Porous N-Doped Carbon for Efficient and Stable Water Splitting
Nafion D521 was purchased from SCI Materials Hub.
10. Molecules Interfacial Interaction in NiFe LDH/NiS2/VS2 for Enhanced Electrocatalytic Water Splitting
Carbon cloth (SCI Materials Hub) were employed as substrates for the in-situ formation of VS2 and NiS2/VS2 on its surface via hydrothermal synthesis.
11. Chemical Engineering Journal Mapping hydrogen evolution activity trends of V-based A15 superconducting alloys
Carbon fiber paper (GDS250) was obtained from the SCI materials Hub.
12. Advanced Science A Dual-Cation Exchange Membrane Electrolyzer for Continuous H2 Production from Seawater
The CEMs include GORE-SELECT Gore M788.12 (W. L. Gore & Associates, America) and FUMA Fumasep FKB-PK-130 (FuMa Tech., Co., Ltd., Germany) were provided by SCI Materials Hub.
13. Ind. Eng. Chem. Res. Electrolysis of Tertiary Water Effluents - a Pathway to Green Hydrogen
The PEM electrolyzer stack PSC2000 was purchased from the SCI Materials Hub with a maximum hydrogen production capability of 2000 mL/min. The stack had 8 electrolysis cells with a maximum recommended operation current of 36 A and a voltage of 24 V. Its membrane electrode assembly had an effective area of 56 cm2 per layer and a catalyst loading of 4.0 mg/cm2 on Nafion 117 for Ir black as anode and Pt/C as cathode, respectively. The catalysts were deposited on the Nafion membrane to form a catalyst-coated membrane. Titanium bipolar plates were used to construct the electrolyzer. Water is supplied to the anode side of the electrolyzer stack during operation.
14. Adv. Energy Mater. High-Efficiency Iridium-Yttrium Alloy Catalyst for Acidic Water Electrolysis
Carbon paper (Toray TGP-H-060) was purchased from the SCI Materials Hub.
15. Journal of Alloys and Compounds Amorphous/Crystalline Nife Ldh Hierarchical Nanostructure for Large-Current-Density Electrocatalytic Water Oxidation
The commercial NiFe foam (NFF) was offered by SCI Materials Hub.
W0S1009 Carbon cloth (CC, SCI Materials Hub) were employed as substrate for the in-situ formation of Ru-VS2 and VS2 on its surface via hydrothermal synthesis.
17. Journal of Colloid and Interface Science The dual active sites reconstruction on gelatin in-situ derived 3D porous N-doped carbon for efficient and stable overall water splitting
Nafion D521 was purchased from SCI Materials Hub.
18. Journal of Physics and Chemistry of Solids AgCo bimetallic cocatalyst modified g-C3N4 for improving photocatalytic hydrogen evolution
Nafion D520 dispersion (5 wt%) was purchased from SCI Materials Hub.
19. Separation and Purification Technology NiP2 as an efficient non-noble metal cathode catalyst for enhanced hydrogen isotope separation in proton exchange membrane water electrolysis
Ni supported on Vulcan XC-72, obtained from SCI materials Hub.
20. ACS Appl. Nano Mater. Rapid Electrical-Field-Enhanced Corrosion Endows Ni3Fe/NiFe Layered Double Hydroxide Nanosheets with High-Rate Oxygen Evolution Activity
The Ni3Fe substrate obtained directly from a commercial NiFe foam (nominal Ni 70% at. % + Fe 30 at. %, thickness: 2 mm, porosity: 100 PPI, SCI Materials Hub) was cleaned with acetone, ultrapure water, and ethanol successively and was dried with compressed air.
The proposed thin V-Zirfon separator samples were evaluated at first by water electrolysis at 60 °C using a two-compartment zero-gap electrolyzer (LSCF Alkaline Water Electrolyzer stack [1 cell], purchased from SCI Materials Hub).
22. ACS Materials Lett. Promoting Nonacid Hydrogen Evolution over Ni4Mo/Cu by D-Band Regulation
Commercial Pt/C (20%) from Wuhu Eryi Material Technology Co., Ltd.
Note: Wuhu Eryi Material Technology Co. is a company hold by SCI Materials Hub.
23. Molecules Interfacial Interaction in NiFe LDH/NiS2/VS2 for Enhanced Electrocatalytic Water Splitting
Carbon cloth (CC, SCI Materials Hub) were employed as substrates for the in-situ formation of VS2 and NiS2/VS2 on its surface via hydrothermal synthesis.
24. Nat. Commun. Flexible tungsten disulfide superstructure engineering for efficient alkaline hydrogen evolution in anion exchange membrane water electrolysers
Commercial IrO2, Pt/C (40 wt%), anion exchange membrane (Sustainion X37-50 Grade 60) and carbon fiber cloth (CFC) were obtained from SCI Materials Hub.
25. International Journal of Hydrogen Energy Enhancing performance of anion exchange membrane electrolyzer through modification of carbon paper liquid-gas diffusion layer
The anode is made of Ni–Fe foam (60% Fe + 40% Ni, SCI Materials Hub, China), while the cathode is made of carbon paper electrode. AEM employed is the highly stable PiperION™-A40-HCO3 (with a thickness of 40 μm).
26. Nature Communications Rationally designed Ru catalysts supported on TiN for highly efficient and stable hydrogen evolution in alkaline conditions
Fumasep FAAM-20 anion exchange membrane was purchased from SCI Materials Hub.
27. Nature Communications Redox-mediated decoupled seawater direct splitting for H2 production
Nickel foam (Ni Foam, aperture: 110 ppi, area density: 380 ± 20 g cm−2), platinum carbon (Pt/C, 20 wt%), anion exchange membranes (FAA-3-PK-75), graphite felt (thickness: ~2 mm), and commercial alkaline water electrolyzers were purchased from SCI Materials Hub.
28. ACS Applied Materials & Interface Promoting Reaction Kinetics of the Air Cathode for Neutral Zinc–Air Batteries by the Photothermal Effect
Carbon paper (SGL Carbon Sigracet 22BB) was purchased from SCI Materials Hub.
29. International Journal of Hydrogen Energy Efficient and stable formaldehyde-polyoxometalate battery for dual-decoupled hydrogen production
Firstly, the surface of the Cu foam (1 × 1 cm2, Sci Materials Hub Co.) was oxidized into Cu(OH)2 nanowires (denoted as sample Cu(OH)2 NWs/Cu foam) by (NH4)2S2O8 (0.13 M) in 2.67 M NaOH aqueous solution for 30 min at room temperature.
30. International Journal of Hydrogen Energy Controlled deposition of trimetallic Fe–Ni–V oxides on nickel foam as high-performance electrocatalysts for oxygen evolution reaction
Nickel foam (95–98% porosity, 40 pores/cm), manufactured by SCI Materials Hub, was employed for catalyst fabrication.
31. Journal of Alloys and Compounds Cobalt-doping mediated low-valence Rh centers in rhodium sulfide superconductor for improved electrocatalytic hydrogen evolution
Carbon fiber paper (Spectracarb 2050 A, thickness of 10μm, density of 0.50 g cm−3) was purchased from SCI Materials Hub.
32. J. Mater. Chem. A Promoted surface reconstruction of amorphous nickel boride electrocatalysts by boron dissolution for boosting oxygen evolution reaction
The ink was also sprayed onto a 1.5 mm-thick Ni foam substrate (SCI Materials Hub, China).
33. Advanced Energy Materials Surface Reconstruction Activates Non-Noble Metal Cathode for Proton Exchange Membrane Water Electrolyzer
34. J. Mater. Chem. A Multimetallic layered double hydroxides as efficient and durable oxygen evolution catalysts for anion exchange membrane water electrolysis at high current densities
35. Nano Research Hierarchical NiFe LDH/N-doped Co/nickel foam as highly active oxygen evolution reaction electrode for anion exchange membrane water electrolysis
Commercial PtRu/C and RuO2 were purchased from SCI Materials Hub.
36. J. Mater. Chem. A Ga doping Enhances Oxygen Evolution Reaction Performance and stability of NiFe layered double hydroxides
Nickel foam (NF) was purchased from SCI Materials Hub (99.9%,bore diameter 200-250um, thickness 0.3mm).
37. Journal of Industrial and Engineering Chemistry Optimizing CoFe2O4 coatings on nickel foam via Aerosol-Assisted CVD for superior electrochemical oxygen evolution Catalysis
To fabricate the catalyst, we used nickel foam (95–98 % porosity, with 40 pores/cm) produced by SCI Materials Hub
38. International Journal of Hydrogen Energy Experimental and simulation study of H2 crossover in PEM water electrolysis for high-pressure hydrogen production up to 20 MPa
The high-pressure PEMWE cell used a PFSA membrane (Nafion® 117, DUPONT) coated with 0.75 mg cm−2 of Pt/C (40 %, JOHNSON MATTHEY) on the cathode and 1 mg cm−2 of IrO2 (Accelerate®, SCI-MATERIALS HUB) on the anode.
39. Journal of Alloys and Compounds Ruthenium-mediated synthesis of ultrafine FePtCoNiRu high-entropy alloy nanoparticles for enhanced hydrogen evolution catalysis
Vulcan XC-72R (Ketjen Black) was purchased from SCI Materials Hub.
40. Nano-Micro Letters A Strongly Coupled Cluster Heterostructure with Pt–N-Mo Bonding for Durable and Efficient H2 Evolution in Anion-Exchange Membrane Water Electrolyzers
Carbon paper and anion-exchange membrane (PiperION A40) were obtained from SCI Materials Hub.
41. Journal of Alloys and Compounds N, P-doped coconut shell activated carbon supported Mo2C catalysts for alkaline water electrolysis hydrogen evolution
Nafion solution (D520, 5 wt%) was obtained from SCI Materials Hub.
42. Nature Communications Lanthanum-assisted lattice anchoring of iridium in Co3O4 for efficient oxygen evolution reaction in low-iridium water electrolysis
Carbon paper was obtained from SCI Materials Hub.
43. Nanoscale Two-dimensional 1T-phase MnxIr1−xO2 for high-performance acidic oxygen evolution reaction
Commercial Nafion NR212 films were purchased from SCI Materials Hub.
44. Journal of Physics: Conference Series Stability and durability of nickel-molybdenum foam electrodes in electrocatalytic seawater splitting
The Materials used in the present investigation were prepared by SCI Materials Hub.
电 催 化
1. Nature Communications Electrochemical epoxidation enhanced by C2H4 activation and hydroxyl generation at the Ag/SnO2 interface
Fumion FAA anion exchange resin and FAA-3-50 membranes with a thickness of 50 µm were purchased from SCI Materials Hub.
燃料电池
1. Polymer Sub-two-micron ultrathin proton exchange membrane with reinforced mechanical strength
Gas diffusion electrode (60% Pt/C, Carbon paper) was purchased from SCI Materials Hub.
Fumion FAA-3-solut-10 was obtained from SCI Materials Hub.
3. Journal of Power Sources Boosting the power density of the H3PO4/polybenzimidazole high-temperature proton exchange membrane fuel cell to >1.2 W cm-2 via the deposition of acid-based polymer layers on the catalyst layers
PBI resin (molecular weight: 60000, SCI Materials Hub), carbon paper 39BB (SGL Carbon), 70 wt% Pt/C (TANAKA) were obtained from SCI Materials Hub.
Fumasep FAA-3-20 was obtained from SCI Materials Hub.
5. ACS Sustainable Chem. Eng. Vanadium-Mediated High Areal Capacity Zinc–Manganese Redox Flow Battery
Zinc plate (thickness 1 mm), copper foam (thickness 1.5 mm), and Ketjenblack (KB) EC-600JD were procured from SCI materials hub.
6. ACS Appl. Energy Mater. Investigation of Pd2B- and NiB-Doped Pd–Ni/C Electrocatalysts with High Activity for Methanol Oxidation
Nafion solution (5 wt %, DuPont) was purchased from SCI Materials Hub.
7. Chemical Engineering Journal Loosened Hydrophobic Microphase to Facilitate Ion Channel Formation in Anion Exchange Membrane for Fuel Cell Applications
Fumasep FAA-3-20 was obtained from SCI Materials Hub.
8. Energy Conversion and Management: X Amplified impact of contact uniformity on the performance of low-catalyst-loading fuel cells
Commercial Pt/C (weight ratio of Pt, 5 %), commercial Pt/C (weight ratio of Pt, 10 %), carbon black (Ketjenblack EC-300 J), the expanded polytetrafluoroethylene (ePTFE) reinforced proton exchange membranes (PEM) (manufactured by GORE), and GDL were purchased from SCI Materials Hub.
9. ACS Sustainable Chemistry & Engineering Lignin-Derived Sustainable Cationic Polymers for Efficient High-Temperature Proton Exchange Membrane Fuel Cells
Poly-2,2′-(m-phenylene)-5,5′-bibenzimidazole (PBI, Figure 1a) [(MW)RU ∼ 308 g/mol, Mw ∼ 60,000 g/mol, Tg ∼ 427 °C] was purchased from SCI Materials Hub (China).
10. Journal of Energy StorageAnion-type solvation structure enables stable zinc‑iodine flow batteries
The Nafion 115 produced by Dupont was purchased from SCI Materials Hub and pretreated by the standard acid boiling procedure.
11. Chemical Engineering Journal A novel biofuel cell based on galactose oxidase and bilirubin oxidase for efficient glycerol conversion and electricity generation
W1S1011 carbon cloth (CC) with PTFE&MPL (CeTech) was purchased from SCI Materials Hub (China).
12. ACS Nano Improving Fuel Cell Performance of FeNx-Based Catalysts by Introducing Graphitic Microdomains in the Carbon Matrix
13. Science Advances Manipulating the coordination dice: Alkali metals directed synthesis of Co-N-C catalysts with CoN4 sites
A Nafion D521 dispersion (5 wt %, equivalent weight = 1100), PtRu-C (60% PtRu on high–surface area carbon, Pt:Ru = 1:1, SCI Materials Hub), an alkaline ionomer dispersion (PiperION-A5-HCO3-EtOH, 5 wt %, Versogen Inc.), and anion exchange membrane (PiperION-A type-HCO3, Versogen Inc.) were obtained from SCI Materials Hub.
催化-ORR
1. J. Chem. Eng. Superior Efficiency Hydrogen Peroxide Production in Acidic Media through Epoxy Group Adjacent to Co-O/C Active Centers on Carbon Black
In this paper, Vulcan XC 72 carbon black, ion membrane (Nafion N115, 127 μL), Nafion solution (Nafion D520, 5 wt%), and carbon paper (AvCarb GDS 2230 and Spectracarb 2050A-1050) were purchased from SCI Materials Hub.
2. Journal of Colloid and Interface Science Gaining insight into the impact of electronic property and interface electrostatic field on ORR kinetics in alloy engineering via theoretical prognostication and experimental validation
The 20 wt% Pt3M (M = Cr, Co, Cu, Pd, Sn, and Ir) were purchased from SCI Materials Hub. This work places emphasis on the kinetics of the ORR concerning Pt3M (M = Cr, Co, Cu, Pd, Sn, and Ir) catalysts, and integrates theoretical prognostication and experimental validation to illuminate the fundamental principles of alloy engineering.
3. Catalysis Solution-Phase Synthesis of Co-N-C Catalysts Using Alkali Metals-Induced N-C Templates with Metal Vacancy-Nx sites
In this paper, PtRu-C (60 % PtRu (3.5nm) on High Surface Area Carbon, Pt:Ru = 1:1, SCI Materials Hub), an alkaline dispersion (PiperION-A5-HCO3-EtOH, 5 wt.%, SCI Materials Hub), anion exchange membrane (PiperION-A type-HCO3, SCI Materials Hub) were used as received.
4. Green Chemistry Low Cell Voltage Electrosynthesis of Hydrogen Peroxide
The proton exchange membranes (Nafion-117, 211, and 212) were from SCI Materials Hub. They were pre-treated by 5 v/v% H2O2 solution for 1 h at 80°C and then treated by 10 v/v% H2SO4 aqueous solution for 1 h at 80°C before assembling to flow cell reactor.
5. Chemosphere Sustainable H2O2 production in a floating dual-cathode electro-Fenton system for efficient decontamination of organic pollutants
Ketogen black (EC-600JD) was purchased from SCI Materials Hub.
6. Journal of Materials Science Carbon dot intercalated MXene with an excellent oxygen reduction reaction electrocatalytic performance
Nafion (5 wt%) was purchased from SCI Materials Hub (Nafion D520).
7. Nature Commuinications Precisely designing asymmetrical selenium-based dual-atom sites for efficient oxygen reduction
Vulcan XC-72R carbon black (CAS No.: 1333-86-4, SCI Materials Hub).
8. ChemElectroChem Balancing the Aggregation of Cobalt Phthalocyanine on Carbon Nanohorn for Efficient H2O2 electrosynthesis in Neutral Electrolyte
AvCarb GDS 2230 substrates and Nafion 1110 membranes were purchased from Sci Materials hub.
Nafion solution (D520, 5 wt%) and proton exchange membrane (PEM, Nafion N117) were purchased from Sci Materials Hub.
Carbon cloth (W0S1011) was purchased from SCI Materials Hub.
11. Nature Portfolio Carbon defects enhanced TEMPO redox cycles for high-efficiency urotropine electrosynthesis
TGPH060H hydrophilic carbon paper (CP), VXC-72 carbon black (CB), nickel foam, Nafion D520 dispersion, Nafion N117, and Fumasep FAA-3-PK-75 were gained from SCI Materials Hub.
12. American Institute of Chemical Engineers A tandem electro-thermocatalysis platform for practical hydrogen peroxide-mediated oxygenation reactions at high rates
Sustainion ionomerbinder solution and anion-exchange membrane (AEM, SustainionX37-50 grade 60) was purchased from SCI Materials Hub.
13. Journal of Environmental Chemical Engineering Metal-free heterogeneous electro-Fenton with solid polymer electrolytes: A novel self-supporting system for PPCPs decontamination
Industrial-grade graphite felt (GF) and solid polymer electrolytes (Nafion®N324, N438, N117, and N115) were procured from SCI Materials Hub.
14. Physical Chemistry Chemical Physics Tailoring LaBO3 (B = Mn, Fe, Co, Ni) perovskite oxides for methanol-tolerant oxygen reduction reaction electrocatalysts
Nafion D520 dispersion (5 wt%) was obtained from SCI Materials Hub.
15. Nano Research Surface diffusion-Limited dealloying: A strategy for porosityconstruction in small-sized alloy nanoparticle electrocatalysts
Commercial 20%Pt/C (TKK-TEC-10E20E) was obtained from SCl MaterialsHub.
电容器
1. Journal of Energy Storage Unraveling the detrimental crosstalk between cathode and anode in the aqueous asymmetric capacitor of activated carbon /copper oxide
In this paper, Fumasep FAA-3-50 anion exchange membrane (Thickness 50 μm, surface resistance 0.6–1.5 Ω cm−2, transference number 92–96 %) was bought from SCI Materials Hub.
2. Composites Science and Technology High modulus carbon fiber based composite structural supercapacitors towards reducing internal resistance and improving multifunctional performance
The aluminium tape (Wuhu Eryi Materials Co. LTD) were used as the current collectors.
Note: Wuhu Eryi Material Technology Co. is a company hold by SCI Materials Hub.
Carbon cloth (CeTech W0S1011) was sourced from SCI Materials Hub.
4. ACS Applied Materials & Interfaces Green Polymer Derived Multifunctional Layer Achieving Oriented Diffusion and Controllable Deposition of Zn2+ for Ultra-Durable Zinc-Ion Hybrid Supercapacitors
Zn foil (99.99%) and Copper foil (99.99%) was purchased from SCI Materials Hub.
5. Journal of Water Process Engineering Enhanced electrodialysis by electric double-layer capacitors to minimize membrane use in digestate ammonia recovery: mechanisms and effects
Carbon cloth (W0S1011, >98 %), cation-exchange membrane (FKB-PK-130, with a thickness of 130 μm) were purchased from SCI Materials Hub.
6. ACS Applied Materials & Interfaces Mechano-Electrochemical Synergy of Lignin Cross-Linked PVA Gel Polymer Electrolytes for Wide-Temperature Flexible Supercapacitors
Raw carbon cloth (CeTech W0S1009) was acquired from SCI Materials Hub
催化加氢
1. Nature Communications Electrosynthesis of polymer-grade ethylene via acetylene semihydrogenation over undercoordinated Cu nanodots
In this paper, activated carbon (Vulcan XC-72) was obtained from SCI Materials Hub.
2. Applied Catalysis B: Environment and Energy Spatial-confined effect of CuOx microneedles bundles on TiO2 nanotubes: Reinforcing the adsorption and enrichment of ultralow concentration nitrate for efficient NH3 electrosynthesis
Ion membrane (Nafion N115, 127 μL) was purchased form Sci Materials Hub.
3. Nature Communications Electrosynthesis of NH3 from NO with ampere-level current density in a pressurized electrolyzer
The anion exchange membrane (Fumasep FAB-PK-130, 130 μm) was purchased from SCI Materials Hub.
4. Sustainable Energy Fuels Dibenzyl ether-guided microstructural regulation of PtIrZn catalysts for ammonia electrocatalysis
5. Chemical Engineering Journal Enhanced electrocatalytic nitrate reduction to ammonia via boron-doped copper foam
Ni foam was purchased from the Sci Materials Hub Co., Ltd. (Anhui, China).
6. Nature Communications Modulating Ru-Co bond lengths in Ru1Co single-atom alloys through crystal phase engineering for electrocatalytic nitrate-to-ammonia conversion
Nafion 211 membrane (diameter: 3 cm, thickness: 25.4 μm) was purchased from SCI Materials Hub.
7. Nature Communications Revealing and modulating catalyst reconstruction for highly efficient electrosynthesis of ammonia
Toray Carbon Paper (CP, TGP-H-060), Nafion 211 membrane, Nafion N117 membrane, and membrane electrode device were obtained from the Sci materials hu
Hydrophilic carbon paper and carbon black, carbon rod electrode was purchased from SCI Materials HUB
9. Separation and Purification Technology A novel membrane-free ion enrichment electrolyzer recovers ammonia from digestate
Carbon cloth (W0S1011, >98 %), cation-exchange membrane (FKB-PK-130, with a thickness of 130 μm), and anion-exchange membrane (FAS-PET-130, with a thickness of 130 μm) were purchased from SCI Materials Hub.
水处理
1. Water Research Electro-peroxone with solid polymer electrolytes: A novel system for degradation of plasticizers in natural effluents
In this paper, Nafion® N324 (SCI Materials Hub), between a 15 cm2 (3 cm × 5 cm) graphite plate anode and a graphite felt cathode (EP-SPE system)
表征
1. Chemical Engineering Journal Electrochemical reconstitution of Prussian blue analogue for coupling furfural electro-oxidation with photo-assisted hydrogen evolution reaction
An Au nanoparticle film was deposited on the total reflecting plane of a single reflection ATR crystal (SCI Materials Hub, Wuhu, China) via sputter coater.
理论计算
1. Sustainable Energy & Fuels A desulfurization fuel cell with alkali and sulfuric acid byproducts: a prototype and a model
A Fumasep®FKD-PK-75 membrane was used as the cation exchange membrane, in which the the oxygen permeability of membrane was about 1 cm3(STP)/(s cm2 cm Hg) [Ref. SCI Materials Hub]
器件
1. Journal of Materials Science: Materials in Electronics Preparation and application of electrical conductive composites with skin temperature-triggered attachable and on-demand detachable adhesion
Carbon black (CB, Ketjenblack EC 600JD) was purchased from SCI Materials Hub.
2. Chemosphere Highly Sensitive Electrochemical Sensor for Lead Ion Based on Bi-Mof/Conducting Polymer Composites
Nafion D520 Dispersion (Alcohol based 1000 EW at 5wt%) was purchased from SCI Materials Hub.
3. ACS Nano High-Performance Graphdiyne Oxide/Au Nanoparticle Electrode for Electrochemical Non-enzymatic Glucose Sensor
Carbon cloth was purchased from SCI Materials Hub (CeTech W0S1011).
材料合成
1. Acta Materialia In situ epitaxial thickening of wafer-scale, highly oriented nanotwinned Ag on tailored polycrystalline Cu substrates
Single-crystal Cu (1 cm × 1 cm) substrates with a (111) orientation were purchased from SCI Materials Hub.
2. Journal of The Electrochemical Society One-Pot Electrodeposition of a PANI:PSS/MWCNT Nanocomposite on Carbon Paper for Scalable Determination of Ascorbic Acid
Raw carbon paper was purchased from SCI Materials Hub
3. Polymer Composites Constructing High-Performance Composite Bipolar Plates via Multidimensional Carbon Networks
KB (EC-600JD), Acetylene black (CB11), and Carbon black (XC-72) were purchased from SCI Materials Hub
催化降解
1. Journal of Environmental Chemical Engineering Conversion of CoNiFe-LDH to CoNiFe-MOF/LDH as catalyst for efficient heterogeneous electro-Fenton degradation of sulfonamide antibiotics
The hydrophobic microporous laminated carbon paper (HML-CP) (2 cm × 2.5 cm) was chosen as a cathode and fabricated by Wuhu Eryi Material Technology Co. (Anhui, China).
Note: Wuhu Eryi Material Technology Co. is a company hold by SCI Materials Hub.
2. Nature Communications Scale-up upcycling of waste polyethylene terephthalate plastics to biodegradable polyglycolic acid plastics
Nickle foam (NF) and Nafion N117 were obtained from SCI Materials Hub.
催化电解
1. Chemical Engineering Journal Modulation of energy barrier of reaction steps over S-doped Ni(OH)2/Cu composites to achieve high-performance urea electrolysis catalysts
Commercial Pt/C (20 wt%) was purchased from Wuhu Eryi Material Technology Co., LTD.
Note: Wuhu Eryi Material Technology Co. is a company hold by SCI Materials Hub.
2. Chemical Engineering Journal Efficient Catalysis for Acidic Methanol Oxidation: Exploration of a Low-Platinum Quaternary Alloy Catalyst Via a Two-Step Method
Nafion (5%) was purchased from SCI Materials Hub.
环 境
1. Journal of Materials Research and TechnologyTribocorrosion performance of TC4 anodized/carbon fiber composite in marine environment
Carbon fiber cloth WOS1011H(M) purchased from Wuhu Eryi Materials Technology Co.
Note: Wuhu Eryi Material Technology Co. is a company hold by SCI Materials Hub.
2. Adv. Funct. Mater. Modulating NFO@N-MWCNTs/CC Interfaces to Construct Multilevel Synergistic Sites (Ni/Fe-O-N-C) for Multi-Heavy Metal Ions Sensing
Carbon cloth (W0S1011) was acquired from SCI Materials Hub (www.scimaterials.cn).
热 电
1. Chemical Engineering Journal High power density charging-free thermally regenerative electrochemical flow cycle for low-temperature thermoelectric conversion
The heat exchangers are composed of 20 μm thick titanium foil (SCI Materials Hub), 1 mm thick rubber gasket and 2 cm thick organic glass from the inside to the outside.
其 它
1. Ceramics International Superhydrophobic carbon fiber composite coatings based on TC4 titanium alloy for improving corrosion resistance
Carbon fiber cloth was purchased from SCI Materials Hub.
2. Electrochimica Acta Integrated electrochemically assisted absorbers for the removal of Carbon dioxide
39 BB carbon paper was obtained from SCI Materials Hub.
3. Journal of Hazardous Materials Chain assembly of Rhodococcus bacteria with O-doped g-C3N4 for photocatalysis mediated high-performance partial nitrification: From nitrite resource evolution to device application
Fumasep FAA-3-PK-130, diameter 30 mm, was purchased from SCI Materials Hub.
4. Nature Communications High selectivity framework polymer membranes chemically tuned towards fast anion conduction
Sustainion® X37-50 Grade RT membrane was purchased from SCI Materials Hub, and was pretreated by soaking in 1 M KOH for 24 h according to a reported procedure.
5. Polymer Composites Constructing High-Performance Composite Bipolar Plates via Multidimensional Carbon Networks
KB (EC-600JD), Acetylene black (CB11), and Carbon black (XC-72) were purchased from SCI Materials Hub Co. Ltd. China.

|
为科学研究提供来源广泛的材料 材料合成仪器装置及材料解决方案 备案号:皖ICP备2021011042号-1 |
关于我们 |



