
1. 简介
阴离子交换膜(AEM)是一种通常由离聚物制成的半透膜,设计用于传导阴离子,同时不渗透诸如氧气或氢气的气体。阴离子交换膜的主要优点是使用非铂/低铂催化剂,在达到相同性能的前提下,可以大大降低成本,阴离子交换膜的其他优点还包括:耐碱、抗氧化、耐氯离子、扩散透析以回收酸。
科学材料站可以提供Xion AEM-Pention-18-5CL不同厚度尺寸系列,其中厚度有5μm, 10μm, 20μm, 30μm和50μm,尺寸有5x5cm, 10x10cm及15x15cm。更多型号将在厂家更新后提供。
如需购买请点进入方【购买渠道】进行购买或寻求报价单。
Xion-Pention复合阴离子交换膜是一系列复合阴离子交换膜(AEM),使用基于聚降冰片烯的树脂的复合而成,复合材料来自于与膜结构整合的增强层,用以提高其机械性能,机械性能的增强可以使复合膜比独立膜更薄,在不牺牲强度的情况下提供更高的离子导电性,其离子交换容量(IEC)为3.4-3.6meq/g,这些特制的阴离子交换膜依其厚度和交联度而定,已在碱性燃料电池中显示出高达9A/cm2的电流密度(或大于3 W/cm2的功率密度),具有出色的耐久性和寿命(以上数据基于美国可再生能源实验室、南卡罗来纳大学和乔治亚理工学院联合开展的研究,研究原文见文末或本站下载中心,所述的电化学性能数据仅供参考,并取决于MEA、CCM或GDE制造方法、使用的膜厚度、测试硬件设计和测试硬件中使用的组件以及操作参数(温度、压力、反应物流速等),这些值可能会或可能不会达到。)
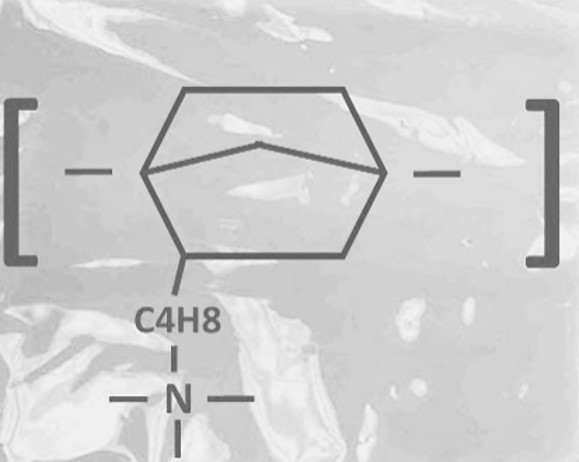
季铵改性聚降冰片烯聚合物的化学成分如下图所示。

2. Xion-Pention聚合物
Xion-Pention复合阴离子交换膜的优点:
√ 高阴离子电导率
√ 在低温和高温下具有良好的化学稳定性
√ 具有优异机械强度的超薄膜
科学材料站目前提供72系列、35系列、18系列,交联度(Crosslinking)为5%和15%,厚度为5um、10um、20um、30um、50um厚度,尺寸为5cmX5cm、10cmX10cm、15cmX15cm、20cmX20cm的Xion-Pention阴离子交换膜片,由于所有这些膜产品都是经过机械增强的,因此特别适合于不加压或加压的阴离子交换膜燃料电池(带或不带碱性电解质),碱性燃料电池(带或不带碱性电解质),碱子泵,碱性氧浓缩器,碱性低氧和碱性电池或其他各种中性至碱性电化学装置。可以使用气态或液态反应物而不会出现任何问题。
选购说明:
1.当您的装置需要拥有最佳的效率并且整个膜上不会有太大的压差时。或者当反应物(燃料或氧化剂)以干燥气体(0%RH)或部分加湿(小于50%RH)的形式提供时。选用厚度较薄的膜(小于10um)可以在整个膜上提供出色的水传输,在阳极和阴极均保持足够的水合作用,因此,当燃料或氧化剂以干态供应时,过氧化氢自由基和其他对膜有害的自由基种类的产生减至最少或部分加湿。
2.当您的装置需要拥有很长的使用寿命,或者整个膜上有较大的压差,又或是装置以足够潮湿的状态(大于50% RH)提供反应物(燃料或氧化剂)时。您需要选用较厚的膜(大于10um)。
性能参数如下所示:
复合膜类型 | 阴离子膜/碱性膜AEM |
复合膜型号 | 72系列、35系列、18系列 |
复合膜厚度 | 5/10/20/30/50 um |
复合膜尺寸(支持定制) | 5X5cm、10X10cm、15X15cm、20X20cm |
IEC | 3.4-3.6 meq/g |
交联度 | 5%或者15% |
吸水率(取决于交联度) | 60-100 wt% |
拉伸强度(在50%RH,50℃下测量) | 40-50 MPa |
OH-电导率(在80℃时测量,取决于交联度) | 125-180 mS/cm |
预处理方案:
复合Pention膜以溴化物或氯化物形式运输。
1.当用于标准碱性燃料电池/电解池时,应通过用0.5-1.0 M NaOH或KOH溶液处理将膜转化为OH-形式:将膜样品置于0.5–1.0 M NaOH或KOH的水溶液中,在20°C–30°C下放置24小时。用去离子水(pH≈7)冲洗后,膜即可使用。请使用密闭容器以避免CO2污染(碳酸盐的形成可能会影响电导率)。氢氧根形式的膜必须在潮湿/加湿且不含CO2的条件下保存,避免氢氧根形式的膜变干。在干燥条件下的长期储存应优选以碳酸盐,氯离子或溴离子形式进行。
2.当用于其他电化学(电渗析,脱盐,电渗析,反向电渗析,酸回收,盐分离等)和非电化学应用时,应将膜转换为与预期应用相关的阴离子形式。例如,如果应用程序要求将氯离子通过膜转移,则该阴离子交换膜需要转化为氯离子形式。为了将该膜转化为氯离子形式,需要将其浸入1-2 M NaCl或KCl盐溶液(溶于去离子水中)24-72小时,然后用去离子水冲洗以除去膜表面多余的盐。或者,如果预期的应用需要转移硫酸根阴离子,则该阴离子交换膜需要先预处理成硫酸根形式,在室温下将膜完全浸入盐溶液中24-72小时后,Na2SO4或K22SO4的中性盐溶液通常足以使膜完全转化为硫酸盐形式。
如果您对存储,化学稳定性,预处理或在进行操作之前有任何疑问,请随时与我们联系以获取更多信息。
| AEM-Pention-18-5CL (5, 10, 20 30, 50μm) 阴离子交换膜 - SCI Materials Hub | |
| Membrane Thickness | 5, 10, 20, 30, 50μm |
| Polymer Type | Poly(norbornene) based backbone |
| Functional Group | Quaternary ammonium cationic groups as functional groups |
| Counter ion | Halide (Cl- or Br-) |
| Mechanical Reinforcement | Yes, ePTFE (also known as expanded PTFE) is the mechanical reinforcement substrate |
| Self-supporting Membrane | No |
| Ion Exchange Capacity | 3.4 - 3.6 meq/g |
| Ionic Conductivity | Undisclosed |
| Molecular Weight | Undisclosed |
| Water Uptake | Undisclosed |
| Melting Point | Undisclosed |
| Decomposition Point | Undisclosed |
| pH range | 1 - 14. With alkaline electrolytes, concentrations greater than 1 - 1.5M should not be used with this membrane. |
The Xion Pention-AEM-18 membrane is a composite AEM that uses the poly(norbornene) based resin and has an ion exchange capacity of 3.4 to 3.6 mequiv/g. Pention anion exchange membranes offer excellent mechanical strength and stability to a wide variety of chemistries. These particular AEMs depending on their thickness and crosslinking degree have demonstrated up to 9 A/cm2 current densities (or >3 W/cm2 power densities) in alkaline fuel cells with excellent durability and lifetime [based on the recent jointly conducted study between NREL, University of South Carolina, and Georgia Institute of Technology].
The stated electrochemical performance data is for reference only and depending on the MEA, CCM or GDE manufacturing method, used membrane thickness, testing hardware design and components used in the test hardware, and operational parameters (temperature, pressure, reactant flow rates, etc.), those values may or may not be attained.
SCI Materials Hub currently provides Composite Pention-18-5CL series anion exchange membrane sheets in 5, 10, 20, 30 and 50μm thicknesses and 5x5cm, 10x10cm, and 15x15cm sizes. Image on the bottom shows the chemical composition of the anion exchange resin used to manufacture Xion Composite Pention membranes.
The Xion Composite Pention-AEM-18 series can be used in fuel cells, electrolyzers, electrodialysis, redox flow batteries, electrochemical compressors, and a wide variety of other devices. Composite Pention AEMs are currently offered with 5% or 15% crosslinking levels.
XION Composite PENTION Membranes are ultra-thin, ultra-strong, and provide ultra-high performance while demonstrating highly stable performance as reinforced anion exchange membranes (AEM). The ionomer structure contains a poly(norbornene) backbone with quaternary ammonium functional groups. A reinforcement layer is integrated into the structure of the membrane to provide enhanced mechanical properties. The enhanced mechanical properties as free-standing membranes, providing higher ionic conductance without sacrificing strength.
-High anionic conductivity
-Great chemical stability at low and high temperatures
-Ultra-thin membranes with excellent mechanical strength
The membrane is delivered in dry form with the counter anion being either in bromide or chloride. Depending on application and cell design, assembling is possible in dry form (without pretreatment) or wet form.
For standard alkaline fuel cell / electrolysis applications, the membrane should be converted into OH-form by treating it with 0.5 – 1.0 M NaOH or KOH solution: Put the membrane sample in an aqueous solution of 0.5 – 1.0 M NaOH or KOH for at least 24 h at 20°C – 30°C. After rinsing with demineralised water (pH ~ 7) the membrane is ready to use. Use closed container to avoid CO2 contamination (carbonate formation that may affect conductivity). The membrane in OH-form must be stored under wet / humidified and CO2-free conditions, avoid drying out of the membrane in OH-form. Long-term storage in dry conditions should be preferably done in carbonate, Cl- or Br-form.
For electrochemical CO2 reduction applications, the anion exchange membrane should be converted to the carbonate or bicarbonate form by treating the membrane initially with 0.1 to 0.5 M KOH or NaOH solution and then with 0.1 to 0.5 M water soluble carbonate or bicarbonate salt solutions (such as potassium carbonate or potassium bicarbonate that is dissolved in de-ionized water or distilled water). Fully submerging the anion exchange membrane into KOH or NaOH solution for 6 to 12 hours and then to the desired carbonate or bicarbonate salt solution for a period of 48-72 hours would be sufficient to fully convert the membrane into either carbonate or bicarbonate form. After rinsing the membrane (which is in the carbonate form) with deionized water or distilled water, it can be assembled inside the electrochemical setup for electrochemical CO2 reduction experiments. While the submersion of the membrane into the KOH or NaOH can be skipped, for such situations, a longer submersion time may be required in order to fully convert the membrane to carbonate or bicarbonate form. Initial conversion to OH- form significantly improves the carbonate ion exchange process due to expanded pore sizes.
For other electrochemical (electrodialysis, desalination, electro-electrodialysis, reverse electrodialysis, acid recovery, salt splitting, etc.) and non-electrochemical applications, the membrane should be converted into the anionic form that is relevant for the intended application. For example, if the application is requiring the Cl- anions to be transferred through the membrane, then this anion exchange membrane needs to be converted into the Cl- form. In order to convert this membrane into Cl- form, it needs to be submerged into a 1-2 M salt solution of NaCl or KCl (dissolved in deionized water) for a period of 24-72 hours and then rinsed with deionized water to remove the excess salt from the membrane surface. Or if the intended application is requiring to transfer sulfate anions, then this anion exchange membrane needs to be converted into the sulfate form prior to its assembly into the cell. A neutral salt solution of Na2SO4 or K2SO4 would usually be sufficient to achieve the full conversion of membrane into the sulfate form after fully submerging the membrane into the salt solution for 24-72 hours at room temperature.
If you have any concerns about storage, chemical stability, pre-treatment or before proceeding, please feel free to contact us for further information.
1. 手机淘宝(官方淘宝店铺:科学材料站)
2. 点击进入淘宝网页链接
| 科学材料站 XION AEM碱性阴离子膜系列 | ||||||||
| 产品描述 | 厚度 | 产品代码 | 5*5cm | 10*10cm | 15*15cm | 20*20cm | 其它尺寸及价格 | 备注 |
| AEM-Durion-G2 | 5μm | 1801001 | -- | -- | -- | -- | -- | LMW系列 |
| 10μm | 1801002 | -- | -- | -- | -- | -- | ||
| 20μm | 1801003 | -- | -- | -- | -- | -- | ||
| 30μm | 1801004 | -- | -- | -- | -- | -- | ||
| AEM-Pention-72-5CL | 5μm | 1801005 | ¥1731 | -- | -- | -- | -- | 72系列-5%交联度 |
| 10μm | 1801006 | ¥1661 | -- | -- | -- | -- | ||
| 20μm | 1801007 | ¥1569 | -- | -- | -- | -- | ||
| 30μm | 1801008 | ¥1523 | -- | -- | -- | ¥ (15x18cm) ¥ (17x18cm) | ||
| 50μm | 1801009 | ¥2284 | -- | -- | -- | ¥ (15x16cm) ¥ (16x16cm) | ||
| AEM-Pention-72-15CL | 5μm | 1801010 | -- | -- | -- | -- | -- | 72系列-15%交联度 |
| 10μm | 1801011 | -- | -- | -- | -- | -- | ||
| 20μm | 1801012 | -- | -- | -- | -- | ¥ (16CM*16CM) | ||
| 30μm | 1801013 | -- | -- | -- | -- | ¥ (5CM*7.8CM) ¥ (6.5CM*9.5CM) ¥ (11CM*16CM) | ||
| AEM-Pention-35-5CL | 5μm | 1801014 | -- | -- | -- | -- | -- | 35系列-5%交联度 |
| 10μm | 1801015 | -- | -- | -- | -- | -- | ||
| 20μm | 1801016 | -- | -- | -- | -- | -- | ||
| 30μm | 1801017 | -- | -- | -- | -- | -- | ||
| 50μm | 1801018 | -- | -- | -- | -- | -- | ||
| AEM-Pention-35-15CL | 5μm | 1801019 | -- | -- | -- | -- | -- | 35系列-15%交联度 |
| 10μm | 1801020 | -- | -- | -- | -- | -- | ||
| 20μm | 1801021 | -- | -- | -- | -- | -- | ||
| 30μm | 1801022 | -- | -- | -- | -- | -- | ||
| AEM-Pention-18-5CL | 5μm | 1801023 | -- | -- | -- | -- | -- | 18系列-5%交联度 |
| 10μm | 1801024 | -- | -- | -- | -- | -- | ||
| 20μm | 1801025 | -- | -- | -- | -- | -- | ||
| 30μm | 1801026 | -- | -- | -- | -- | -- | ||
| 50μm | 1801027 | -- | -- | -- | -- | -- | ||
| AEM-Pention-18-15CL | 5μm | 1801028 | -- | -- | -- | -- | -- | 18系列-15%交联度 |
| 10μm | 1801029 | -- | -- | -- | -- | -- | ||
| 20μm | 1801030 | -- | -- | -- | -- | -- | ||
| 30μm | 1801031 | -- | -- | -- | -- | -- | ||
| AEM-Dappion | 5μm | 1801032 | -- | -- | -- | -- | -- | Dappion系列 |
| 10μm | 1801033 | -- | -- | -- | -- | -- | ||
| 20μm | 1801034 | -- | -- | -- | -- | -- | ||
| 30μm | 1801035 | -- | -- | -- | -- | -- | ||
| SCI Materials Hub Is Committed to Offering The Best Price & Customer Services! | ||||||||
注:下单时请联系客服,注明选择哪个系列(72系列、35系列、18系列)和哪种交联度(5%CL、15%CL)
3. 其它联系方式
电话:+86 130-0303-8751/+86 156-0553-2352
微信:SCI-Materials-Hub
如需报价单请联系:Email: contact@scimaterials.cn
部分使用和引用我们材料的参考文献 (摘自于Google Scholar)
二氧化碳还原
1. ACS Nano Strain Relaxation in Metal Alloy Catalysts Steers the Product Selectivity of Electrocatalytic CO2 Reduction
The bipolar membrane (Fumasep FBM) in this paper was purchased from SCI Materials Hub, which was used in rechargeable Zn-CO2 battery tests. The authors reported a strain relaxation strategy to determine lattice strains in bimetal MNi alloys (M = Pd, Ag, and Au) and realized an outstanding CO2-to-CO Faradaic efficiency of 96.6% with outstanding activity and durability toward a Zn-CO2 battery.
2. Front. Chem. Boosting Electrochemical Carbon Dioxide Reduction on Atomically Dispersed Nickel Catalyst
In this paper, Vulcan XC-72R was purchased from SCI Materials Hub. Vulcan XC 72R carbon is the most common catalyst support used in the anode and cathode electrodes of Polymer Electrolyte Membrane Fuel Cells (PEMFC), Direct Methanol Fuel Cells (DMFC), Alkaline Fuel Cells (AFC), Microbial Fuel Cells (MFC), Phosphoric Acid Fuel Cells (PAFC), and many more!
3. Adv. Mater. Partially Nitrided Ni Nanoclusters Achieve Energy-Efficient Electrocatalytic CO2 Reduction to CO at Ultralow Overpotential
An AEM membrane (Sustainion X37-50 Grade RT), purchased from SCI Materials Hub) was activated in 1 M KOH for 24 h, washed with ultra-purity water prior to use.
4. Adv. Funct. Mater. Nanoconfined Molecular Catalysts in Integrated Gas Diffusion Electrodes for High-Current-Density CO2 Electroreduction
In this paper (Supporting Information), an anion exchanged membrane (Fumasep FAB-PK-130 obtained from SCI Materials Hub (www.scimaterials.cn)) was used to separate the catholyte and anolyte chambers.
SCI Materials Hub: we also recommend our Fumasep FAB-PK-75 for the use in a flow cell.
5. Appl. Catal. B Efficient utilization of nickel single atoms for CO2 electroreduction by constructing 3D interconnected nitrogen-doped carbon tube network
In this paper, the Nafion 117 membrane was obtained from SCI Materials Hub.
In this paper, Proton exchange membrane (Nafion 117), Nafion D520, and Toray 060 carbon paper were purchased from SCI Materials Hub.
7. National Science Review Confinement of ionomer for electrocatalytic CO2 reduction reaction via efficient mass transfer pathways
An anion exchange membrane (PiperION-A15-HCO3) was obtained from SCI Materials Hub.
8. Catalysis Communications Facilitating CO2 electroreduction to C2H4 through facile regulating {100} & {111} grain boundary of Cu2O
Carbon paper (TGPH060), membrane solution (Nafion D520), and ionic membrane (Nafion N117) were obtained from Wuhu Eryi Material Technology Co., Ltd.
Note: Wuhu Eryi Material Technology Co. is a company hold by SCI Materials Hub.
9. Advanced Energy Materials Interatomic Electronegativity Offset Dictates Selectivity When Catalyzing the CO2 Reduction Reaction
The bipolar membrane (Fumasep FBM), carbon paper (SIGRACET 29BC, Freudenberg paper H23C2), ion exchange membrane (Nafion N117), and anion exchange membrane (Fumasep, FAA-3-PK-130) were all obtained from SCI Materials Hub.
10. Separation and Purification Technology *CO spillover induced by bimetallic xZnO@yCuO active centers for enhancing C–C coupling over electrochemical CO2 reduction
5 % Nafion solution was obtained through SCI Materials Hub.
11. National Science Review Confinement of ionomer for electrocatalytic CO2 reduction reaction via efficient mass transfer pathways
In this paper, PiperION-A5-HCO3 anion exchange resin, Fumion FAA anion exchange resin, PiperION-A15-HCO3 and FAA-3-50 were purchased from SCI Materials Hub.
12. Vacuum Controllable dual Cu–Cu2O sites derived from CuxAl-LDH for CO2 electroreduction to hydrocarbons
Nafion and carbon paper (TGPH060) were supplied through SCI Materials Hub.
13. Chemical Engineering Journal Coupling electrocatalytic CO2 reduction with glucose oxidation for concurrent production of formate with high efficiency
An AEM membrane (PiperION, purchased from SCI Materials Hub) was activated in 1 M KOH for 24 h, washed with ultra-purity water prior to use.
In this paper, Sustainion X37-50 Grade RT membrane and the MEA electrolyzer (CRRMEA1a, Figure S34) with 1cm2 active area were obtained from SCI Materials Hub.
微信公众号中文报道:Chem:基于热力学驱动的混合策略形成Cu0/Cu+/Cu0界面用于中性条件CO2电还原C2+
15. Surfaces and Interfaces Modulating surface microenvironment based on Ag-adorned CuO flower-liked nanospheres for strengthening C-C coupling during CO2RR
5 wt.% of Nafion solution, and N115 proton exchange membrane were procured with the help of SCI Materials Hub
16. ACS Appl. Energy Mater. Nanoporous Bismuth Induced by Surfactant-Modified Dealloying for Efficient Electrocatalytic Reduction of CO2 to Formic Acid
The anion exchange membrane (AEM, PiperION A20) and cation exchange membrane (CEM, Nafion 117) were obtained from SCI Materials Hub.
17. Adv. Energy Mater. Tailoring Microenvironments and In Situ Transformations of Cu Catalysts for Selective and Stable Electrosynthesis of Multicarbon Products
For GDE-based CO₂ electrolysis, the MEA reactor (CRRMEA5a, Sci-Materials Hub) consists of a titanium anode plate and a cathode plate with flow fields, along with insulating gaskets, integrated into a compression cell. The geometric area of each flow field is 5 cm² An anion exchange membrane (PiperION, A40-HCO3, Versogen) was used to separate the anode and the cathode.
18. Journal of Environmental Chemical Engineering Evaluation of electromethanogenesis in a microbial electrolysis cell using nylon cloth as a separator: reactor performance and metagenomic analysis
A commercial Nafion PEM (SCI Materials Hub) was used as the control to compare the electromethanogenesis performance.
CRRMEA1a 1cm2 MEA electrolyzer (Figure 4d) was obtained from SCI Materials Hub.
微信公众号中文报道:安徽师范大学最新Angew!全pH范围内铋基催化剂用于安培级电流密度电催化CO2还原
20. Separation and Purification Technology Coupling regulation of boron doping and morphology in nano-floral CuO using one pot method for electrocatalytic CO2 reduction
Carbon paper (TGPH060), Dupont Nafion solution (D520), and proton exchange membrane (N117) were acquired by SCI Materials Hub.
21. Chemical Engineering Journal Manipulating dual effects of morphology and oxygen vacancies through the incorporation of CuO onto CeO2 nanospheres for electrochemical CO2 reduction
Carbon paper (TGPH060), Dupont Nafion solution (D520), and proton exchange membrane (N117) were acquired by SCI Materials Station Hub (SCI Materials Hub, the same author as Ref. 20).
22. Advanced Materials Universal Formation of Single Atoms from Molten Salt for Facilitating Selective CO2 Reduction
The Nafion D520 dispersion and gas diffusion electrode (GDE, Sigracet, 39BB) were obtained from SCI Materials Hub (www.scimaterials.cn).
23. Science Bulletin Compressive strain in Cu catalysts: Enhancing generation of C2+ products in electrochemical CO2 reduction
CRRMEA1a 1cm2 MEA electrolyzer (Figure 3a) was obtained from SCI Materials Hub.
微信公众号中文报道:Science Bulletin:优化水覆盖度促进安培级电流密度下CO2还原C2+
Carbon paper (SGL CARBON, SGL36BB, purchased from SCI Materials Hub)
25. Chemical Engineering Journal In-situ reconstruction of active bismuth for enhanced CO2 electroreduction to formate
Anion-exchange membrane (Fumasep FAA-3–50) was purchased from SCI Materials Hub.
26. Nature Communications Bromide-mediated membraneless electrosynthesis of ethylene carbonate from CO2 and ethylene
Nafion D520 polymer binder solution (5 wt%) was purchased from SCI Materials Hub.
27. Ionics Sulfur-modified promoting the electrochemical CO2 reduction into formate performance of BiOI
Carbon black (Vulcan XC- 72, Cabot), teflon-treated carbon paper (TGP-H- 060, Toray), and proton exchange membrane (Nafion- 117) were purchased from SCI Materials Hub.
28. Nature Communications Continuous decoupled redox electrochemical CO2 capture
The anion exchange membrane (FAA-3-PK-75) with a thickness of 75 ± 5 μm was purchased from SCI Materials Hub.
29. Journal of Energy Chemistry Construction of efficient electrodes for CO2RR through microenvironment regulation of hydrophobic ionomer
An anion exchange membrane (PiperION-A15-HCO3, SCI Materials Hub) was used to separate the anode and cathode chambers.
30. Journal of Energy Chemistry The insights into ionomer-catalyst interactions enabling high-efficiency CO2 electroreduction in pure water
Methylimidazolium ionomer powder (Sustainion XA-9, dispersed in ethanol at 5 wt%), and piperidinium ionomer solution (PiperION A5, dispersed in ethanol at 5 wt%) were purchased from SCI Materials Hub (
31. ChemElectroChem Scaling CO Electrolyzers for Carbon-Neutral Chemical Synthesis
Specifically, the PTFE-CB ink was prepared by mixing 0.1 g of CB (Vulcan XC-72R, SCI Materials Hub) with PTFE (25 % of the CB mass) in 50 mL of methanol. After ultrasonication for 2 hours, the ink was air-sprayed onto the gas diffusion layer (GDL) (Sigracet 28 BC, SCI Materials Hub) at 60 °C. The anion exchange membrane (AEM) was Fumasep FAA-3-50 (SCI Materials Hub)
32. American Institute of Chemical Engineers Porous carbon-supported Ag catalysts enablehigh-current-density CO2 electroreductionto CO with enhanced mass transport
Carbon paper (SGL Sigracet 39 BB SGL Carbon), anion exchange membrane (Sustainion X37-50 Grade T, Dioxide Materials), Ni foam was obtained from SCI Materials Hub.
33. Applied Catalysis B: Environment and Energy Microenvironmental hydrophobicity-regulated evolution of reaction sites for boosting electrochemical CO2 reduction
A gas diffusion layer AvCarb GDS2240 was obtained from SCI MATERIALS HUB.
34. Applied Catalysis B: Environment and Energy Boosting seawater desalination driven by electroreduction of CO2 to formate on bimetallic Sn/Cu polyphthalocyanine frameworks
Gas diffusion layers (GDLs) consisting of Sigracet 36BB substrates (SCI Materials Hub) were utilized.
35. Catalysis Today Efficient and cost-effective electrocatalysts for coproduction of formate through electrocatalytic oxidation of PET-derived ethylene glycol coupled with CO2 reduction
Cation exchange membrane ((Nafion 117, DuPont) was purchased from SCI Materials Hub, China.
电池
1. J. Mater. Chem. A Blocking polysulfides with a Janus Fe3C/N-CNF@RGO electrode via physiochemical confinement and catalytic conversion for high-performance lithium–sulfur batteries
Graphene oxide (GO) in this paper was obtained from SCI Materials Hub. The authors introduced a Janus Fe3C/N-CNF@RGO electrode consisting of 1D Fe3C decorated N-doped carbon nanofibers (Fe3C/N-CNFs) side and 2D reduced graphene oxide (RGO) side as the free-standing carrier of Li2S6 catholyte to improve the overall electrochemical performance of Li-S batteries.
This paper used more than 10 kinds of materials from SCI Materials Hub and the authors gave detailed properity comparsion.
The commercial IEMs of Fumasep FAB-PK-130 and Nafion N117 were obtained from SCI Materials Hub.
Gas diffusion layers of GDL340 (CeTech) and SGL39BC (Sigracet) and Nafion dispersion (Nafion D520) were obtained from SCI Materials Hub.
Zn foil (100 mm thickness) and Zn powder were obtained from the SCI Materials Hub.
Commercial 20% Pt/C, 40% Pt/C and IrO2 catalysts were also obtained from SCI Materials Hub.
3. Journal of Energy Chemistry Vanadium oxide nanospheres encapsulated in N-doped carbon nanofibers with morphology and defect dual-engineering toward advanced aqueous zinc-ion batteries
In this paper, carbon cloth (W0S1011) was obtained from SCI Materials Hub. The flexible carbon cloth matrix guaranteed the stabilization of the electrode and improved the conductivity of the cathode.
4. Energy Storage Materials Defect-abundant commercializable 3D carbon papers for fabricating composite Li anode with high loading and long life
The 3D carbon paper (TGPH060 raw paper) were purchased from SCI Materials Hub.
5. Nanomaterials A Stable Rechargeable Aqueous Zn–Air Battery Enabled by Heterogeneous MoS2 Cathode Catalysts
Nafion D520 (5 wt%), and carbon paper (GDL340) were received from SCI-Materials-Hub.
Carbon cloth (W0S1011) and other electrochemical consumables required for air cathode were provided by SCI Materials Hub.
The Zn sheet (99.99%) was purchased from SCI Materials Hub.
8. Nature Communications Atomic-scale regulation of anionic and cationic migration in alkali metal batteries
The lithium metal disk (purity: 99.9%, diameter: 16 mm, thickness: 0.6 mm) was obtained from SCI Materials Hub.
9. Chemical Engineering Journal Zinc-based energy storage with functionalized carbon nanotube/polyaniline nanocomposite cathodes
CNTs were purchased from SCI Materials Hub.
10. ACS Nano Interfacial Chemistry Modulation via Amphoteric Glycine for a Highly Reversible Zinc Anode
Zn foil (>99.99%, 100 μm) was purchased from SCI Materials Hub.
11. ACS Nano High-Energy and Long-Lived Zn–MnO2 Battery Enabled by a Hydrophobic-Ion-Conducting Membrane
Zn foil (99.9%), carbon paper, and carbon felt were obtained from SCI Materials Hub.
12. Nature Communications Unravelling rechargeable zinc-copper batteries by a chloride shuttle in a biphasic electrolyte
Carbon cloth (CeTech W0S1011), PP membrane (Celgard 2300), Glass fiber (Whatman GF/A), anion exchange membrane (Fumasep FAB-PK-130), and cation exchange membrane (Nafion N-117) were purchased from sci materials hub.
13. PROCEEDINGS OF SPIE A dendrite-free and corrosion-suppressive metallic Zn anode regulated by the hybrid aqueous/organic electrolyte
Zn foil (99.9%, 100 μm thickness) was obtained from the SCI Materials Hub.
14. Journal of Alloys and Compounds Cr-induced enhancement of structural stability in δ-MnO2 for aqueous Zn-ion batteries
The Zn sheet (99.99%) and Whatman GF/D paper were available for purchase on on the SCI Materials Hub.
Carbon coating aluminum foils with a thickness of 16 µm were acquired from SCI Materials Hub.
16. Journal of Industrial and Engineering Chemistry Investigation into electrochemical catalytic properties and electronic structure of Mn doped SrCoO3 perovskite catalysts
KB-EC600JD superconducting carbon black was obtained from SCI Materials Hub.
17. Journal of Alloys and Compounds Inhibiting polysulfide shuttle and enhancing polysulfide redox: Conductive 2D metal-organic framework coated separators for lithium-sulfur batteries
Ketjen black was obtained from SCI Materials Hub
18. Chemical Engineering Journal Regulating N-doped biochar with Fe-Mo heterojunctions as cathode in long-life zinc-air battery
Conductive carbon black (Vulcan XC-72R) was purchased from SCI Materials Hub (Wuhu, Anhui).
19. Nature Portfolio Fast-kinetics and high-compatibility aqueous cadmium-metal battery for next-generation energy storage infrastructures (Under review.)
Cd foil (99.9%), Zn foil (99.9%) and polytetrafluoroethylene (PTFE) aqueous dispersion solution were obtained from the supplier of SCI Materials Hub.
All cells were assembled using two-electrode Swagelok-type configurations, supported by SCI Materials Hub.
Celgard 2400 separator (polypropylene (PP), 25 μm) was sourced from SCI Materials Hub.
21. ACS Applied Energy Materials 3D Graphene Nanoflake/Vertically Aligned Carbon Nanotube/CoAl Layered Double Oxide Composites for High-Performance Lithium-Ion Batteries
Carbon-coated aluminum foil was procured from SCI Materials Hub.
22. Chemistry A European Jouranl Regulating the Interfacial Charge Density by Constructing a Novel Zn Anode-Electrolyte Interface for Highly Reversible Zn Anode
Ketjenblack (KB) was purchased from SCI Materials Hub
23. Advanced Functional Materials A Biphasic Membraneless Zinc-Iodine Battery with High Volumetric Capacity
Graphite felt (GF, G650A) was purchased from SCI Materials Hub.
24. Science Advances Alcohol molecule coupling: A universal approach to modulating amorphousness in vanadium-based cathodes for high-rate and durable aqueous zinc-ion batteries
Fueiceel® BM03 Battery test hardware in Figure 6F was obtained from SCI Materials Hub. The Fueiceel® BM03 scale-up battery powering an LED panel.
25. Nature Communications Cooperative Jahn-Teller effect and engineered long-range strain in manganese oxide/graphene superlattice for aqueous zinc-ion batteries
The Zn||MnO2 coin cells were assembled by Zn foil (thickness of 0.1 mm, 99.99%, SCI Materials Hub) counter electrodes
26. Adv. Funct. Mater. Construction of Ionic Conductive Electrode/ Electrolyte Interphases via Li+ Coordination Regulator for 4.7 V Li/ LiNi0.9Co0.05Mn0.05O2 Batteries
The CR2032 coin cells were assembled with a Celgard 2400 separator (SCI Materials Hub.) in a glove box.
27. Journal of Energy Storage Bacterial cellulose mixed with glass fiber as a separator to achieve long cycle life of aqueous zinc-ion batteries
304 stainless steel foil (99.99 % purity, purchased from SCI Materials Hub)
28. Nature Communications Dual-structure-breaking electrolyte enables practical cadmium-metal battery
Two-electrode (Swagelok2E002) and three-electrode (Swagelok3E2e) Swagelok-type configurations were supported by SCI Materials Hub (Supplementary Figs. 102, 103)
The stacked configuration (BM03) for the scaled-up cell was supported by SCI Materials Hub.
Cd foil (99.9%), Zn foil (99.9%), and polytetrafluoroethylene (PTFE) aqueous dispersion solution (D-210C, solid content: 60 w%, size: ~0.25 μm size) were obtained from the supplier of SCI Materials Hub.
29. Nature Nanotechnology A nanoengineered lithium-hosting carbon/zinc oxide composite electrode material for efficient non-aqueous lithium metal batteries
The operando battery pressure sensing equipment (BS01) was purchased from SCI Materials Hub (www.scimaterials.cn).
Nafion 115 membrane (N115) was purchased from SCI Materials Hub.
电解水
1. International Journal of Hydrogen Energy Gold as an efficient hydrogen isotope separation catalyst in proton exchange membrane water electrolysis
The cathodic catalysts of Pt/C (20 wt%, 2–3 nm) and Au/C (20 wt%, 4–5 nm) were purchased from SCI Materials Hub.
2. Small Science Silver Compositing Boosts Water Electrolysis Activity and Durability of RuO2 in a Proton-Exchange-Membrane Water Electrolyzer
Two fiber felts (0.35 mm thickness, SCI Materials Hub) were used as the porous transport layers at both the cathode and the anode.
3. Advanced Functional Materials Hierarchical Crystalline/Amorphous Heterostructure MoNi/NiMoOx for Electrochemical Hydrogen Evolution with Industry-Level Activity and Stability
Anion-exchange membrane (FAA-3-PK-130) was obtained from SCI Materials Hub.
4. Chemical Engineering Journal Electronic configuration of single ruthenium atom immobilized in urchin-like tungsten trioxide towards hydrazine oxidation-assisted hydrogen evolution under wide pH media
The non-reinforced anion exchange membrane (AEM) of the coupled system was obtained from SCI Materials Hub (Fumasep FAA-3-50).
5. Cell Reports Physical Science Non-layered dysprosium oxysulfide as an electron-withdrawing chainmail for promoting electrocatalytic oxygen evolution
Nickel foam (NF) was offered by SCI Materials Hub (Wuhu, China), and was ultrasonicated in HCl solution, ethanol, and acetone in proper order before being used in electrochemical measurements.
6. Materials Today Catalysis Valence engineering via double exchange interaction in spinel oxides for enhanced oxygen evolution catalysis
Commercial Cu foam was purchased from SCI Materials Hub.
7. Advanced Functional Materials Elucidating the Critical Role of Ruthenium Single Atom Sites in Water Dissociation and Dehydrogenation Behaviors for Robust Hydrazine Oxidation-Boosted Alkaline Hydrogen Evolution
The nonreinforced anion exchange membrane (AEM) of the HzOR-assisted OWS system was purchased from SCI Materials Hub (Fumasep FAA-3-50).
8. ACS Omega Boosting Hydrogen Evolution through the Interface Effects of Amorphous NiMoO4–MoO2 and Crystalline Cu
Pt/C (20 wt %) was purchased from SCI Materials Hub.
9. Journal of Colloid and Interface Science The Dual Active Sites Reconstruction on Gelatin In-Situ Derived 3d Porous N-Doped Carbon for Efficient and Stable Water Splitting
Nafion D521 was purchased from SCI Materials Hub.
10. Molecules Interfacial Interaction in NiFe LDH/NiS2/VS2 for Enhanced Electrocatalytic Water Splitting
Carbon cloth (SCI Materials Hub) were employed as substrates for the in-situ formation of VS2 and NiS2/VS2 on its surface via hydrothermal synthesis.
11. Chemical Engineering Journal Mapping hydrogen evolution activity trends of V-based A15 superconducting alloys
Carbon fiber paper (GDS250) was obtained from the SCI materials Hub.
12. Advanced Science A Dual-Cation Exchange Membrane Electrolyzer for Continuous H2 Production from Seawater
The CEMs include GORE-SELECT Gore M788.12 (W. L. Gore & Associates, America) and FUMA Fumasep FKB-PK-130 (FuMa Tech., Co., Ltd., Germany) were provided by SCI Materials Hub.
13. Ind. Eng. Chem. Res. Electrolysis of Tertiary Water Effluents - a Pathway to Green Hydrogen
The PEM electrolyzer stack PSC2000 was purchased from the SCI Materials Hub with a maximum hydrogen production capability of 2000 mL/min. The stack had 8 electrolysis cells with a maximum recommended operation current of 36 A and a voltage of 24 V. Its membrane electrode assembly had an effective area of 56 cm2 per layer and a catalyst loading of 4.0 mg/cm2 on Nafion 117 for Ir black as anode and Pt/C as cathode, respectively. The catalysts were deposited on the Nafion membrane to form a catalyst-coated membrane. Titanium bipolar plates were used to construct the electrolyzer. Water is supplied to the anode side of the electrolyzer stack during operation.
14. Adv. Energy Mater. High-Efficiency Iridium-Yttrium Alloy Catalyst for Acidic Water Electrolysis
Carbon paper (Toray TGP-H-060) was purchased from the SCI Materials Hub.
15. Journal of Alloys and Compounds Amorphous/Crystalline Nife Ldh Hierarchical Nanostructure for Large-Current-Density Electrocatalytic Water Oxidation
The commercial NiFe foam (NFF) was offered by SCI Materials Hub.
W0S1009 Carbon cloth (CC, SCI Materials Hub) were employed as substrate for the in-situ formation of Ru-VS2 and VS2 on its surface via hydrothermal synthesis.
17. Journal of Colloid and Interface Science The dual active sites reconstruction on gelatin in-situ derived 3D porous N-doped carbon for efficient and stable overall water splitting
Nafion D521 was purchased from SCI Materials Hub.
18. Journal of Physics and Chemistry of Solids AgCo bimetallic cocatalyst modified g-C3N4 for improving photocatalytic hydrogen evolution
Nafion D520 dispersion (5 wt%) was purchased from SCI Materials Hub.
19. Separation and Purification Technology NiP2 as an efficient non-noble metal cathode catalyst for enhanced hydrogen isotope separation in proton exchange membrane water electrolysis
Ni supported on Vulcan XC-72, obtained from SCI materials Hub.
20. ACS Appl. Nano Mater. Rapid Electrical-Field-Enhanced Corrosion Endows Ni3Fe/NiFe Layered Double Hydroxide Nanosheets with High-Rate Oxygen Evolution Activity
The Ni3Fe substrate obtained directly from a commercial NiFe foam (nominal Ni 70% at. % + Fe 30 at. %, thickness: 2 mm, porosity: 100 PPI, SCI Materials Hub) was cleaned with acetone, ultrapure water, and ethanol successively and was dried with compressed air.
The proposed thin V-Zirfon separator samples were evaluated at first by water electrolysis at 60 °C using a two-compartment zero-gap electrolyzer (LSCF Alkaline Water Electrolyzer stack [1 cell], purchased from SCI Materials Hub).
22. ACS Materials Lett. Promoting Nonacid Hydrogen Evolution over Ni4Mo/Cu by D-Band Regulation
Commercial Pt/C (20%) from Wuhu Eryi Material Technology Co., Ltd.
Note: Wuhu Eryi Material Technology Co. is a company hold by SCI Materials Hub.
23. Molecules Interfacial Interaction in NiFe LDH/NiS2/VS2 for Enhanced Electrocatalytic Water Splitting
Carbon cloth (CC, SCI Materials Hub) were employed as substrates for the in-situ formation of VS2 and NiS2/VS2 on its surface via hydrothermal synthesis.
24. Nat. Commun. Flexible tungsten disulfide superstructure engineering for efficient alkaline hydrogen evolution in anion exchange membrane water electrolysers
Commercial IrO2, Pt/C (40 wt%), anion exchange membrane (Sustainion X37-50 Grade 60) and carbon fiber cloth (CFC) were obtained from SCI Materials Hub.
25. International Journal of Hydrogen Energy Enhancing performance of anion exchange membrane electrolyzer through modification of carbon paper liquid-gas diffusion layer
The anode is made of Ni–Fe foam (60% Fe + 40% Ni, SCI Materials Hub, China), while the cathode is made of carbon paper electrode. AEM employed is the highly stable PiperION™-A40-HCO3 (with a thickness of 40 μm).
26. Nature Communications Rationally designed Ru catalysts supported on TiN for highly efficient and stable hydrogen evolution in alkaline conditions
Fumasep FAAM-20 anion exchange membrane was purchased from SCI Materials Hub.
27. Nature Communications Redox-mediated decoupled seawater direct splitting for H2 production
Nickel foam (Ni Foam, aperture: 110 ppi, area density: 380 ± 20 g cm−2), platinum carbon (Pt/C, 20 wt%), anion exchange membranes (FAA-3-PK-75), graphite felt (thickness: ~2 mm), and commercial alkaline water electrolyzers were purchased from SCI Materials Hub.
28. ACS Applied Materials & Interface Promoting Reaction Kinetics of the Air Cathode for Neutral Zinc–Air Batteries by the Photothermal Effect
Carbon paper (SGL Carbon Sigracet 22BB) was purchased from SCI Materials Hub.
29. International Journal of Hydrogen Energy Efficient and stable formaldehyde-polyoxometalate battery for dual-decoupled hydrogen production
Firstly, the surface of the Cu foam (1 × 1 cm2, Sci Materials Hub Co.) was oxidized into Cu(OH)2 nanowires (denoted as sample Cu(OH)2 NWs/Cu foam) by (NH4)2S2O8 (0.13 M) in 2.67 M NaOH aqueous solution for 30 min at room temperature.
30. International Journal of Hydrogen Energy Controlled deposition of trimetallic Fe–Ni–V oxides on nickel foam as high-performance electrocatalysts for oxygen evolution reaction
Nickel foam (95–98% porosity, 40 pores/cm), manufactured by SCI Materials Hub, was employed for catalyst fabrication.
31. Journal of Alloys and Compounds Cobalt-doping mediated low-valence Rh centers in rhodium sulfide superconductor for improved electrocatalytic hydrogen evolution
Carbon fiber paper (Spectracarb 2050 A, thickness of 10μm, density of 0.50 g cm−3) was purchased from SCI Materials Hub.
32. J. Mater. Chem. A Promoted surface reconstruction of amorphous nickel boride electrocatalysts by boron dissolution for boosting oxygen evolution reaction
The ink was also sprayed onto a 1.5 mm-thick Ni foam substrate (SCI Materials Hub, China).
33. Advanced Energy Materials Surface Reconstruction Activates Non-Noble Metal Cathode for Proton Exchange Membrane Water Electrolyzer
34. J. Mater. Chem. A Multimetallic layered double hydroxides as efficient and durable oxygen evolution catalysts for anion exchange membrane water electrolysis at high current densities
35. Nano Research Hierarchical NiFe LDH/N-doped Co/nickel foam as highly active oxygen evolution reaction electrode for anion exchange membrane water electrolysis
Commercial PtRu/C and RuO2 were purchased from SCI Materials Hub.
36. J. Mater. Chem. A Ga doping Enhances Oxygen Evolution Reaction Performance and stability of NiFe layered double hydroxides
Nickel foam (NF) was purchased from SCI Materials Hub (99.9%,bore diameter 200-250um, thickness 0.3mm).
37. Journal of Industrial and Engineering Chemistry Optimizing CoFe2O4 coatings on nickel foam via Aerosol-Assisted CVD for superior electrochemical oxygen evolution Catalysis
To fabricate the catalyst, we used nickel foam (95–98 % porosity, with 40 pores/cm) produced by SCI Materials Hub
38. International Journal of Hydrogen Energy Experimental and simulation study of H2 crossover in PEM water electrolysis for high-pressure hydrogen production up to 20 MPa
The high-pressure PEMWE cell used a PFSA membrane (Nafion® 117, DUPONT) coated with 0.75 mg cm−2 of Pt/C (40 %, JOHNSON MATTHEY) on the cathode and 1 mg cm−2 of IrO2 (Accelerate®, SCI-MATERIALS HUB) on the anode.
39. Journal of Alloys and Compounds Ruthenium-mediated synthesis of ultrafine FePtCoNiRu high-entropy alloy nanoparticles for enhanced hydrogen evolution catalysis
Vulcan XC-72R (Ketjen Black) was purchased from SCI Materials Hub.
40. Nano-Micro Letters A Strongly Coupled Cluster Heterostructure with Pt–N-Mo Bonding for Durable and Efficient H2 Evolution in Anion-Exchange Membrane Water Electrolyzers
Carbon paper and anion-exchange membrane (PiperION A40) were obtained from SCI Materials Hub.
41. Journal of Alloys and Compounds N, P-doped coconut shell activated carbon supported Mo2C catalysts for alkaline water electrolysis hydrogen evolution
Nafion solution (D520, 5 wt%) was obtained from SCI Materials Hub.
42. Nature Communications Lanthanum-assisted lattice anchoring of iridium in Co3O4 for efficient oxygen evolution reaction in low-iridium water electrolysis
Carbon paper was obtained from SCI Materials Hub.
43. Nanoscale Two-dimensional 1T-phase MnxIr1−xO2 for high-performance acidic oxygen evolution reaction
Commercial Nafion NR212 films were purchased from SCI Materials Hub.
44. Journal of Physics: Conference Series Stability and durability of nickel-molybdenum foam electrodes in electrocatalytic seawater splitting
The Materials used in the present investigation were prepared by SCI Materials Hub.
电 催 化
1. Nature Communications Electrochemical epoxidation enhanced by C2H4 activation and hydroxyl generation at the Ag/SnO2 interface
Fumion FAA anion exchange resin and FAA-3-50 membranes with a thickness of 50 µm were purchased from SCI Materials Hub.
燃料电池
1. Polymer Sub-two-micron ultrathin proton exchange membrane with reinforced mechanical strength
Gas diffusion electrode (60% Pt/C, Carbon paper) was purchased from SCI Materials Hub.
Fumion FAA-3-solut-10 was obtained from SCI Materials Hub.
3. Journal of Power Sources Boosting the power density of the H3PO4/polybenzimidazole high-temperature proton exchange membrane fuel cell to >1.2 W cm-2 via the deposition of acid-based polymer layers on the catalyst layers
PBI resin (molecular weight: 60000, SCI Materials Hub), carbon paper 39BB (SGL Carbon), 70 wt% Pt/C (TANAKA) were obtained from SCI Materials Hub.
Fumasep FAA-3-20 was obtained from SCI Materials Hub.
5. ACS Sustainable Chem. Eng. Vanadium-Mediated High Areal Capacity Zinc–Manganese Redox Flow Battery
Zinc plate (thickness 1 mm), copper foam (thickness 1.5 mm), and Ketjenblack (KB) EC-600JD were procured from SCI materials hub.
6. ACS Appl. Energy Mater. Investigation of Pd2B- and NiB-Doped Pd–Ni/C Electrocatalysts with High Activity for Methanol Oxidation
Nafion solution (5 wt %, DuPont) was purchased from SCI Materials Hub.
7. Chemical Engineering Journal Loosened Hydrophobic Microphase to Facilitate Ion Channel Formation in Anion Exchange Membrane for Fuel Cell Applications
Fumasep FAA-3-20 was obtained from SCI Materials Hub.
8. Energy Conversion and Management: X Amplified impact of contact uniformity on the performance of low-catalyst-loading fuel cells
Commercial Pt/C (weight ratio of Pt, 5 %), commercial Pt/C (weight ratio of Pt, 10 %), carbon black (Ketjenblack EC-300 J), the expanded polytetrafluoroethylene (ePTFE) reinforced proton exchange membranes (PEM) (manufactured by GORE), and GDL were purchased from SCI Materials Hub.
9. ACS Sustainable Chemistry & Engineering Lignin-Derived Sustainable Cationic Polymers for Efficient High-Temperature Proton Exchange Membrane Fuel Cells
Poly-2,2′-(m-phenylene)-5,5′-bibenzimidazole (PBI, Figure 1a) [(MW)RU ∼ 308 g/mol, Mw ∼ 60,000 g/mol, Tg ∼ 427 °C] was purchased from SCI Materials Hub (China).
10. Journal of Energy StorageAnion-type solvation structure enables stable zinc‑iodine flow batteries
The Nafion 115 produced by Dupont was purchased from SCI Materials Hub and pretreated by the standard acid boiling procedure.
11. Chemical Engineering Journal A novel biofuel cell based on galactose oxidase and bilirubin oxidase for efficient glycerol conversion and electricity generation
W1S1011 carbon cloth (CC) with PTFE&MPL (CeTech) was purchased from SCI Materials Hub (China).
12. ACS Nano Improving Fuel Cell Performance of FeNx-Based Catalysts by Introducing Graphitic Microdomains in the Carbon Matrix
13. Science Advances Manipulating the coordination dice: Alkali metals directed synthesis of Co-N-C catalysts with CoN4 sites
A Nafion D521 dispersion (5 wt %, equivalent weight = 1100), PtRu-C (60% PtRu on high–surface area carbon, Pt:Ru = 1:1, SCI Materials Hub), an alkaline ionomer dispersion (PiperION-A5-HCO3-EtOH, 5 wt %, Versogen Inc.), and anion exchange membrane (PiperION-A type-HCO3, Versogen Inc.) were obtained from SCI Materials Hub.
催化-ORR
1. J. Chem. Eng. Superior Efficiency Hydrogen Peroxide Production in Acidic Media through Epoxy Group Adjacent to Co-O/C Active Centers on Carbon Black
In this paper, Vulcan XC 72 carbon black, ion membrane (Nafion N115, 127 μL), Nafion solution (Nafion D520, 5 wt%), and carbon paper (AvCarb GDS 2230 and Spectracarb 2050A-1050) were purchased from SCI Materials Hub.
2. Journal of Colloid and Interface Science Gaining insight into the impact of electronic property and interface electrostatic field on ORR kinetics in alloy engineering via theoretical prognostication and experimental validation
The 20 wt% Pt3M (M = Cr, Co, Cu, Pd, Sn, and Ir) were purchased from SCI Materials Hub. This work places emphasis on the kinetics of the ORR concerning Pt3M (M = Cr, Co, Cu, Pd, Sn, and Ir) catalysts, and integrates theoretical prognostication and experimental validation to illuminate the fundamental principles of alloy engineering.
3. Catalysis Solution-Phase Synthesis of Co-N-C Catalysts Using Alkali Metals-Induced N-C Templates with Metal Vacancy-Nx sites
In this paper, PtRu-C (60 % PtRu (3.5nm) on High Surface Area Carbon, Pt:Ru = 1:1, SCI Materials Hub), an alkaline dispersion (PiperION-A5-HCO3-EtOH, 5 wt.%, SCI Materials Hub), anion exchange membrane (PiperION-A type-HCO3, SCI Materials Hub) were used as received.
4. Green Chemistry Low Cell Voltage Electrosynthesis of Hydrogen Peroxide
The proton exchange membranes (Nafion-117, 211, and 212) were from SCI Materials Hub. They were pre-treated by 5 v/v% H2O2 solution for 1 h at 80°C and then treated by 10 v/v% H2SO4 aqueous solution for 1 h at 80°C before assembling to flow cell reactor.
5. Chemosphere Sustainable H2O2 production in a floating dual-cathode electro-Fenton system for efficient decontamination of organic pollutants
Ketogen black (EC-600JD) was purchased from SCI Materials Hub.
6. Journal of Materials Science Carbon dot intercalated MXene with an excellent oxygen reduction reaction electrocatalytic performance
Nafion (5 wt%) was purchased from SCI Materials Hub (Nafion D520).
7. Nature Commuinications Precisely designing asymmetrical selenium-based dual-atom sites for efficient oxygen reduction
Vulcan XC-72R carbon black (CAS No.: 1333-86-4, SCI Materials Hub).
8. ChemElectroChem Balancing the Aggregation of Cobalt Phthalocyanine on Carbon Nanohorn for Efficient H2O2 electrosynthesis in Neutral Electrolyte
AvCarb GDS 2230 substrates and Nafion 1110 membranes were purchased from Sci Materials hub.
Nafion solution (D520, 5 wt%) and proton exchange membrane (PEM, Nafion N117) were purchased from Sci Materials Hub.
Carbon cloth (W0S1011) was purchased from SCI Materials Hub.
11. Nature Portfolio Carbon defects enhanced TEMPO redox cycles for high-efficiency urotropine electrosynthesis
TGPH060H hydrophilic carbon paper (CP), VXC-72 carbon black (CB), nickel foam, Nafion D520 dispersion, Nafion N117, and Fumasep FAA-3-PK-75 were gained from SCI Materials Hub.
12. American Institute of Chemical Engineers A tandem electro-thermocatalysis platform for practical hydrogen peroxide-mediated oxygenation reactions at high rates
Sustainion ionomerbinder solution and anion-exchange membrane (AEM, SustainionX37-50 grade 60) was purchased from SCI Materials Hub.
13. Journal of Environmental Chemical Engineering Metal-free heterogeneous electro-Fenton with solid polymer electrolytes: A novel self-supporting system for PPCPs decontamination
Industrial-grade graphite felt (GF) and solid polymer electrolytes (Nafion®N324, N438, N117, and N115) were procured from SCI Materials Hub.
14. Physical Chemistry Chemical Physics Tailoring LaBO3 (B = Mn, Fe, Co, Ni) perovskite oxides for methanol-tolerant oxygen reduction reaction electrocatalysts
Nafion D520 dispersion (5 wt%) was obtained from SCI Materials Hub.
15. Nano Research Surface diffusion-Limited dealloying: A strategy for porosityconstruction in small-sized alloy nanoparticle electrocatalysts
Commercial 20%Pt/C (TKK-TEC-10E20E) was obtained from SCl MaterialsHub.
电容器
1. Journal of Energy Storage Unraveling the detrimental crosstalk between cathode and anode in the aqueous asymmetric capacitor of activated carbon /copper oxide
In this paper, Fumasep FAA-3-50 anion exchange membrane (Thickness 50 μm, surface resistance 0.6–1.5 Ω cm−2, transference number 92–96 %) was bought from SCI Materials Hub.
2. Composites Science and Technology High modulus carbon fiber based composite structural supercapacitors towards reducing internal resistance and improving multifunctional performance
The aluminium tape (Wuhu Eryi Materials Co. LTD) were used as the current collectors.
Note: Wuhu Eryi Material Technology Co. is a company hold by SCI Materials Hub.
Carbon cloth (CeTech W0S1011) was sourced from SCI Materials Hub.
4. ACS Applied Materials & Interfaces Green Polymer Derived Multifunctional Layer Achieving Oriented Diffusion and Controllable Deposition of Zn2+ for Ultra-Durable Zinc-Ion Hybrid Supercapacitors
Zn foil (99.99%) and Copper foil (99.99%) was purchased from SCI Materials Hub.
5. Journal of Water Process Engineering Enhanced electrodialysis by electric double-layer capacitors to minimize membrane use in digestate ammonia recovery: mechanisms and effects
Carbon cloth (W0S1011, >98 %), cation-exchange membrane (FKB-PK-130, with a thickness of 130 μm) were purchased from SCI Materials Hub.
6. ACS Applied Materials & Interfaces Mechano-Electrochemical Synergy of Lignin Cross-Linked PVA Gel Polymer Electrolytes for Wide-Temperature Flexible Supercapacitors
Raw carbon cloth (CeTech W0S1009) was acquired from SCI Materials Hub
催化加氢
1. Nature Communications Electrosynthesis of polymer-grade ethylene via acetylene semihydrogenation over undercoordinated Cu nanodots
In this paper, activated carbon (Vulcan XC-72) was obtained from SCI Materials Hub.
2. Applied Catalysis B: Environment and Energy Spatial-confined effect of CuOx microneedles bundles on TiO2 nanotubes: Reinforcing the adsorption and enrichment of ultralow concentration nitrate for efficient NH3 electrosynthesis
Ion membrane (Nafion N115, 127 μL) was purchased form Sci Materials Hub.
3. Nature Communications Electrosynthesis of NH3 from NO with ampere-level current density in a pressurized electrolyzer
The anion exchange membrane (Fumasep FAB-PK-130, 130 μm) was purchased from SCI Materials Hub.
4. Sustainable Energy Fuels Dibenzyl ether-guided microstructural regulation of PtIrZn catalysts for ammonia electrocatalysis
5. Chemical Engineering Journal Enhanced electrocatalytic nitrate reduction to ammonia via boron-doped copper foam
Ni foam was purchased from the Sci Materials Hub Co., Ltd. (Anhui, China).
6. Nature Communications Modulating Ru-Co bond lengths in Ru1Co single-atom alloys through crystal phase engineering for electrocatalytic nitrate-to-ammonia conversion
Nafion 211 membrane (diameter: 3 cm, thickness: 25.4 μm) was purchased from SCI Materials Hub.
7. Nature Communications Revealing and modulating catalyst reconstruction for highly efficient electrosynthesis of ammonia
Toray Carbon Paper (CP, TGP-H-060), Nafion 211 membrane, Nafion N117 membrane, and membrane electrode device were obtained from the Sci materials hu
Hydrophilic carbon paper and carbon black, carbon rod electrode was purchased from SCI Materials HUB
9. Separation and Purification Technology A novel membrane-free ion enrichment electrolyzer recovers ammonia from digestate
Carbon cloth (W0S1011, >98 %), cation-exchange membrane (FKB-PK-130, with a thickness of 130 μm), and anion-exchange membrane (FAS-PET-130, with a thickness of 130 μm) were purchased from SCI Materials Hub.
水处理
1. Water Research Electro-peroxone with solid polymer electrolytes: A novel system for degradation of plasticizers in natural effluents
In this paper, Nafion® N324 (SCI Materials Hub), between a 15 cm2 (3 cm × 5 cm) graphite plate anode and a graphite felt cathode (EP-SPE system)
表征
1. Chemical Engineering Journal Electrochemical reconstitution of Prussian blue analogue for coupling furfural electro-oxidation with photo-assisted hydrogen evolution reaction
An Au nanoparticle film was deposited on the total reflecting plane of a single reflection ATR crystal (SCI Materials Hub, Wuhu, China) via sputter coater.
理论计算
1. Sustainable Energy & Fuels A desulfurization fuel cell with alkali and sulfuric acid byproducts: a prototype and a model
A Fumasep®FKD-PK-75 membrane was used as the cation exchange membrane, in which the the oxygen permeability of membrane was about 1 cm3(STP)/(s cm2 cm Hg) [Ref. SCI Materials Hub]
器件
1. Journal of Materials Science: Materials in Electronics Preparation and application of electrical conductive composites with skin temperature-triggered attachable and on-demand detachable adhesion
Carbon black (CB, Ketjenblack EC 600JD) was purchased from SCI Materials Hub.
2. Chemosphere Highly Sensitive Electrochemical Sensor for Lead Ion Based on Bi-Mof/Conducting Polymer Composites
Nafion D520 Dispersion (Alcohol based 1000 EW at 5wt%) was purchased from SCI Materials Hub.
3. ACS Nano High-Performance Graphdiyne Oxide/Au Nanoparticle Electrode for Electrochemical Non-enzymatic Glucose Sensor
Carbon cloth was purchased from SCI Materials Hub (CeTech W0S1011).
材料合成
1. Acta Materialia In situ epitaxial thickening of wafer-scale, highly oriented nanotwinned Ag on tailored polycrystalline Cu substrates
Single-crystal Cu (1 cm × 1 cm) substrates with a (111) orientation were purchased from SCI Materials Hub.
2. Journal of The Electrochemical Society One-Pot Electrodeposition of a PANI:PSS/MWCNT Nanocomposite on Carbon Paper for Scalable Determination of Ascorbic Acid
Raw carbon paper was purchased from SCI Materials Hub
3. Polymer Composites Constructing High-Performance Composite Bipolar Plates via Multidimensional Carbon Networks
KB (EC-600JD), Acetylene black (CB11), and Carbon black (XC-72) were purchased from SCI Materials Hub
催化降解
1. Journal of Environmental Chemical Engineering Conversion of CoNiFe-LDH to CoNiFe-MOF/LDH as catalyst for efficient heterogeneous electro-Fenton degradation of sulfonamide antibiotics
The hydrophobic microporous laminated carbon paper (HML-CP) (2 cm × 2.5 cm) was chosen as a cathode and fabricated by Wuhu Eryi Material Technology Co. (Anhui, China).
Note: Wuhu Eryi Material Technology Co. is a company hold by SCI Materials Hub.
2. Nature Communications Scale-up upcycling of waste polyethylene terephthalate plastics to biodegradable polyglycolic acid plastics
Nickle foam (NF) and Nafion N117 were obtained from SCI Materials Hub.
催化电解
1. Chemical Engineering Journal Modulation of energy barrier of reaction steps over S-doped Ni(OH)2/Cu composites to achieve high-performance urea electrolysis catalysts
Commercial Pt/C (20 wt%) was purchased from Wuhu Eryi Material Technology Co., LTD.
Note: Wuhu Eryi Material Technology Co. is a company hold by SCI Materials Hub.
2. Chemical Engineering Journal Efficient Catalysis for Acidic Methanol Oxidation: Exploration of a Low-Platinum Quaternary Alloy Catalyst Via a Two-Step Method
Nafion (5%) was purchased from SCI Materials Hub.
环 境
1. Journal of Materials Research and TechnologyTribocorrosion performance of TC4 anodized/carbon fiber composite in marine environment
Carbon fiber cloth WOS1011H(M) purchased from Wuhu Eryi Materials Technology Co.
Note: Wuhu Eryi Material Technology Co. is a company hold by SCI Materials Hub.
2. Adv. Funct. Mater. Modulating NFO@N-MWCNTs/CC Interfaces to Construct Multilevel Synergistic Sites (Ni/Fe-O-N-C) for Multi-Heavy Metal Ions Sensing
Carbon cloth (W0S1011) was acquired from SCI Materials Hub (www.scimaterials.cn).
热 电
1. Chemical Engineering Journal High power density charging-free thermally regenerative electrochemical flow cycle for low-temperature thermoelectric conversion
The heat exchangers are composed of 20 μm thick titanium foil (SCI Materials Hub), 1 mm thick rubber gasket and 2 cm thick organic glass from the inside to the outside.
其 它
1. Ceramics International Superhydrophobic carbon fiber composite coatings based on TC4 titanium alloy for improving corrosion resistance
Carbon fiber cloth was purchased from SCI Materials Hub.
2. Electrochimica Acta Integrated electrochemically assisted absorbers for the removal of Carbon dioxide
39 BB carbon paper was obtained from SCI Materials Hub.
3. Journal of Hazardous Materials Chain assembly of Rhodococcus bacteria with O-doped g-C3N4 for photocatalysis mediated high-performance partial nitrification: From nitrite resource evolution to device application
Fumasep FAA-3-PK-130, diameter 30 mm, was purchased from SCI Materials Hub.
4. Nature Communications High selectivity framework polymer membranes chemically tuned towards fast anion conduction
Sustainion® X37-50 Grade RT membrane was purchased from SCI Materials Hub, and was pretreated by soaking in 1 M KOH for 24 h according to a reported procedure.
5. Polymer Composites Constructing High-Performance Composite Bipolar Plates via Multidimensional Carbon Networks
KB (EC-600JD), Acetylene black (CB11), and Carbon black (XC-72) were purchased from SCI Materials Hub Co. Ltd. China.

|
为科学研究提供来源广泛的材料 材料合成仪器装置及材料解决方案 备案号:皖ICP备2021011042号-1 |
关于我们 |



